Abstract
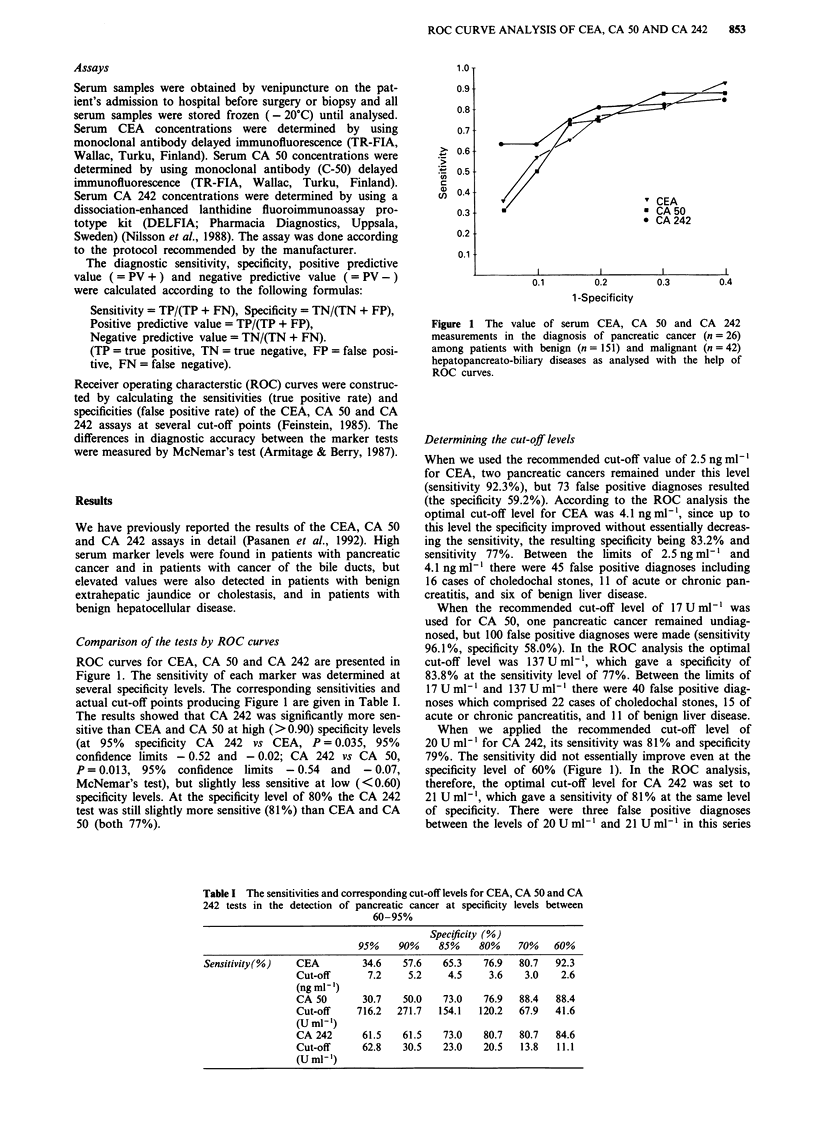
The serum values of the tumour markers carcinoembryonic antigen (CEA), cancer-associated carboanhydrate antigens CA 50 and CA 242 were evaluated in 193 patients with hepatopancreato-biliary diseases by receiver operating characteristic (ROC) curve analysis in order to compare their diagnostic accuracy in pancreatic cancer (n = 26), and to define optimal cut-off levels for the serum values of these tumour markers in the diagnosis of pancreatic cancer. The ROC analysis showed that all marker tests are considerably sensitive (77-81%) at the specificity level of 80%. The CA 242 test was more sensitive than CEA and CA 50 at high specificity levels (> 0.90) but slightly less sensitive at low specificity levels (< 0.60). The CEA test and CA 50 test performed equally well at high and low specificity levels. According to this study, it would seem optimal to use the cut-off level of 4.1 ng ml-1 for CEA, and the level of 137 U ml-1 for CA 50, since they gave a sensitivity of 77% at the specificity levels of 83% and 84%, respectively. For CA 242 the optimal cut-off level was 21 U ml-1, which gave a sensitivity and specificity of 81%. In conclusion, the results of ROC curve analysis suggest that the CA 242 test has an advantage over CEA and CA 50 because of its higher specificity in pancreatic cancer. In addition, it would seem reasonable to use higher cut-off values than what has been recommended for CEA and CA 50 in the diagnosis of pancreatic cancer, but for CA 242 the recommended cut-off level of 20 U ml-1 seems appropriate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begent R. H. The value of carcinoembryonic antigen measurement in clinical practice. Ann Clin Biochem. 1984 Jul;21(Pt 4):231–238. doi: 10.1177/000456328402100401. [DOI] [PubMed] [Google Scholar]
- Carr-Locke D. L. Serum and pancreatic juice carcinoembryonic antigen in pancreatic and biliary disease. Gut. 1980 Aug;21(8):656–661. doi: 10.1136/gut.21.8.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund C., Kuusela P., Jalanko H., Roberts P. J. Serum CA 50 as a tumor marker in pancreatic cancer: a comparison with CA 19-9. Int J Cancer. 1987 Apr 15;39(4):477–481. doi: 10.1002/ijc.2910390412. [DOI] [PubMed] [Google Scholar]
- Haglund C., Lindgren J., Roberts P. J., Kuusela P., Nordling S. Tissue expression of the tumour associated antigen CA242 in benign and malignant pancreatic lesions. A comparison with CA 50 and CA 19-9. Br J Cancer. 1989 Dec;60(6):845–851. doi: 10.1038/bjc.1989.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund C., Roberts P. J., Jalanko H., Kuusela P. Tumour markers CA 19-9 and CA 50 in digestive tract malignancies. Scand J Gastroenterol. 1992;27(3):169–174. doi: 10.3109/00365529208999944. [DOI] [PubMed] [Google Scholar]
- Hansen H. J., Snyder J. J., Miller E., Vandevoorde J. P., Miller O. N., Hines L. R., Burns J. J. Carcinoembryonic antigen (CEA) assay. A laboratory adjunct in the diagnosis and management of cancer. Hum Pathol. 1974 Mar;5(2):139–147. doi: 10.1016/s0046-8177(74)80061-4. [DOI] [PubMed] [Google Scholar]
- Kalser M. H., Barkin J. S., Redlhammer D., Heal A. Circulating carcinoembryonic antigen in pancreatic carcinoma. Cancer. 1978 Sep;42(3 Suppl):1468–1471. doi: 10.1002/1097-0142(197809)42:3+<1468::aid-cncr2820420816>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Haglund C., Roberts P. J. Comparison of a new tumour marker CA 242 with CA 19-9, CA 50 and carcinoembryonic antigen (CEA) in digestive tract diseases. Br J Cancer. 1991 Apr;63(4):636–640. doi: 10.1038/bjc.1991.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Svennerholm L., Fredman P., Nilsson O., Persson B., Myrvold H., Lagergård T. Monoclonal antibodies against gastrointestinal tumour-associated antigens isolated as monosialogangliosides. Int Arch Allergy Appl Immunol. 1983;71(2):178–181. doi: 10.1159/000233384. [DOI] [PubMed] [Google Scholar]
- Lurie B. B., Loewenstein M. S., Zamcheck N. Elevated carcinoembryonic antigen levels and biliary tract obstruction. JAMA. 1975 Jul 28;233(4):326–330. [PubMed] [Google Scholar]
- Masson P., Pålsson B., Andrén-Sandberg A. Cancer-associated tumour markers CA 19-9 and CA-50 in patients with pancreatic cancer with special reference to the Lewis blood cell status. Br J Cancer. 1990 Jul;62(1):118–121. doi: 10.1038/bjc.1990.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O., Johansson C., Glimelius B., Persson B., Nørgaard-Pedersen B., Andrén-Sandberg A., Lindholm L. Sensitivity and specificity of CA242 in gastro-intestinal cancer. A comparison with CEA, CA50 and CA 19-9. Br J Cancer. 1992 Feb;65(2):215–221. doi: 10.1038/bjc.1992.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts P. J. The clinical value of tumour markers. Ann Chir Gynaecol. 1986;75(5):247–248. [PubMed] [Google Scholar]


