Abstract
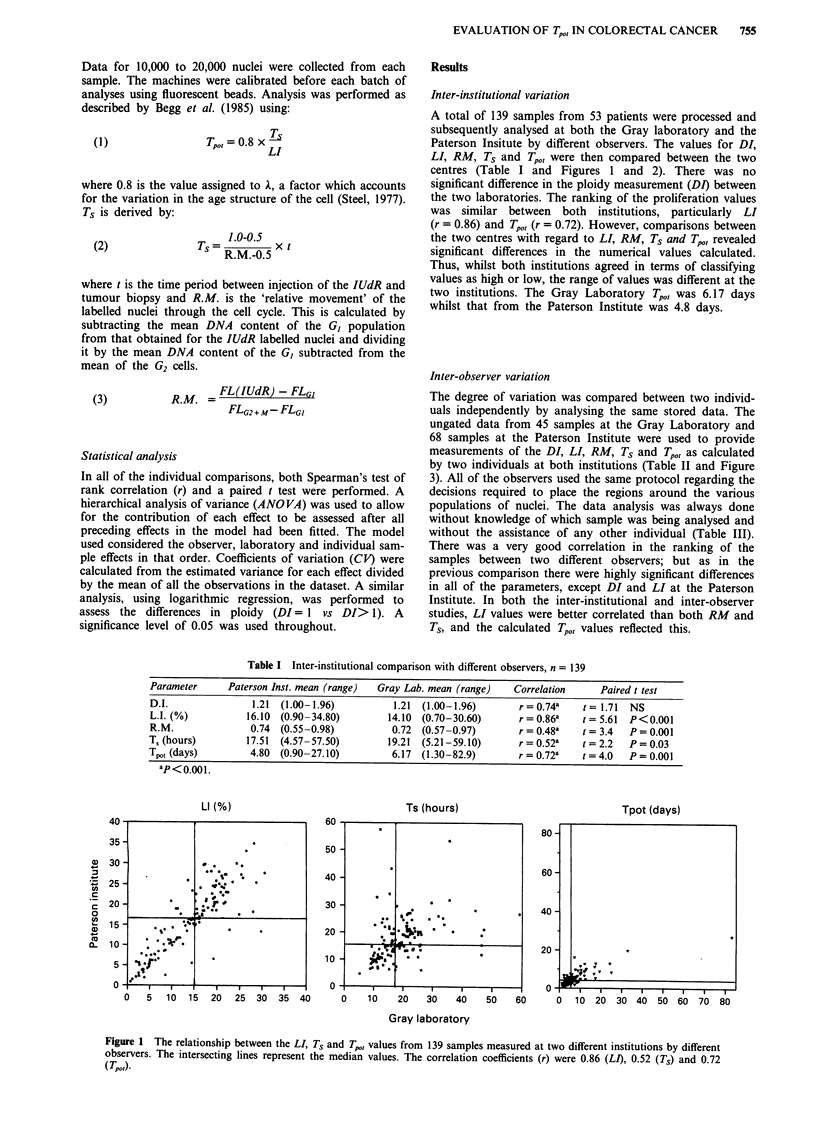
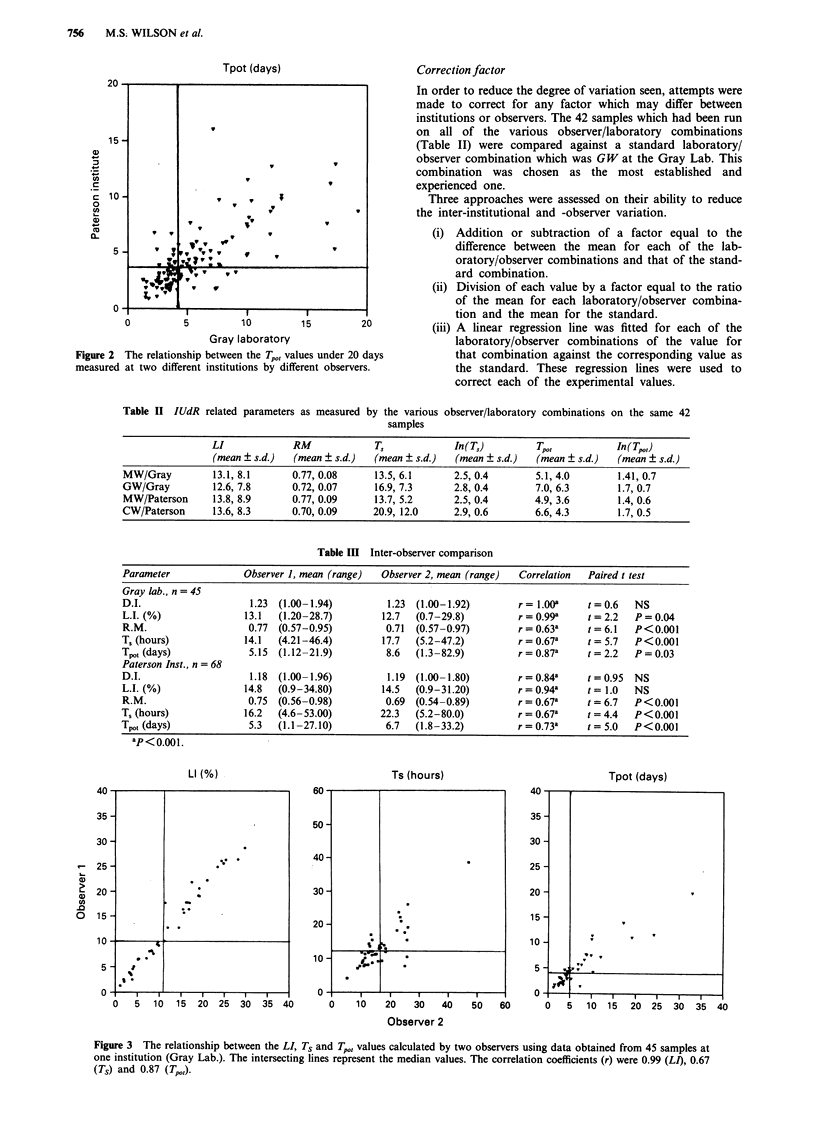
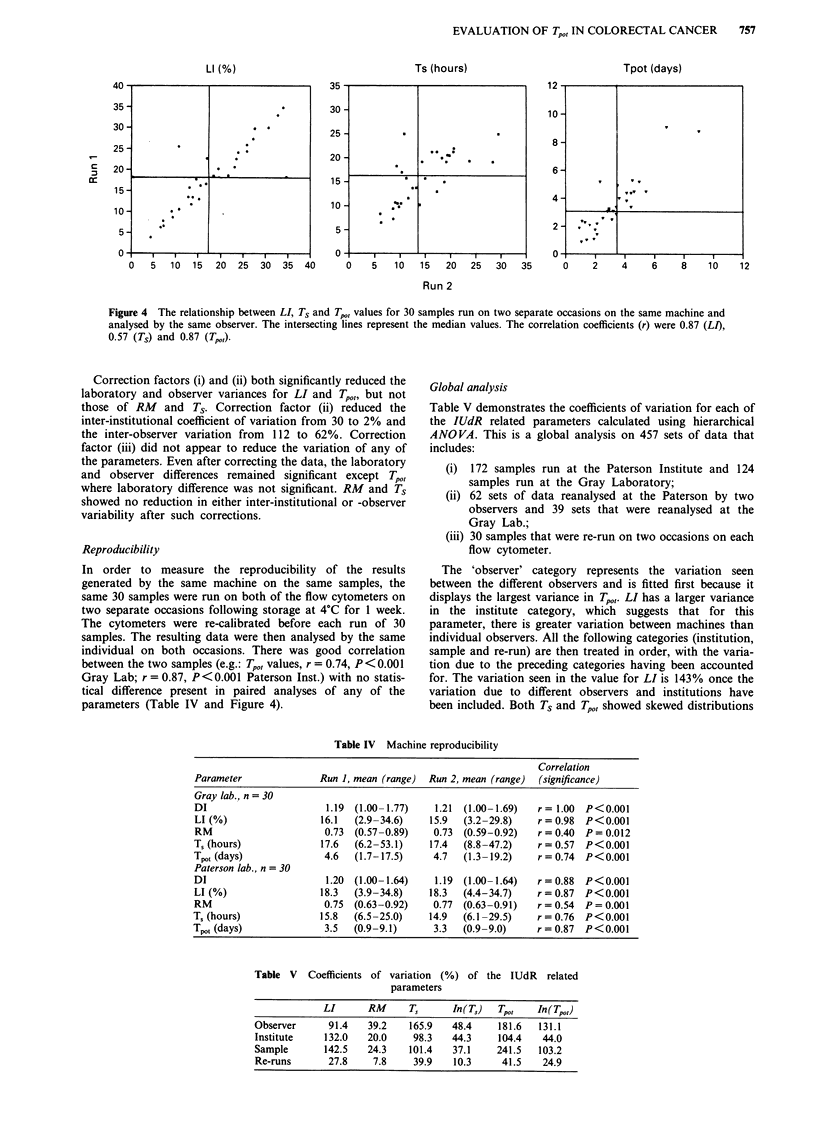
An assessment has been made of the reproducibility of measuring tumour proliferation using in vivo iododeoxyuridine (IUdR) labelling and flow cytometry. The variation that occurs between different institutions (Paterson Institute for Cancer Research, Manchester and the Gray Laboratory, Northwood), different observers and different runs on the same flow cytometer have been measured on 139 samples from 53 patients with colorectal cancer. The results demonstrate that the IUdR technique for measuring tumour proliferation is reproducible. Correlations were seen between measurements of Tpot obtained by different individuals and on separate machines. However, direct comparisons of the measured parameters showed that there were highly significant differences in the values obtained between institutes and observers (P < 0.001). Despite these variations, there were still significant detectable differences in Tpot measurements between individual tumours (P < 0.001). Analysis of the results obtained by running the same samples on two separate occasions on the same machine showed that the technique was highly reproducible and that the staining procedure was stable. Eighty per cent of the samples were similarly assigned to either above or below the median Tpot value, regardless which observer/laboratory combination was utilised. These data suggest that large clinical trials using Tpot should employ a single centre and a single individual to prepare, run and analyse samples.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg A. C., Hofland I., Moonen L., Bartelink H., Schraub S., Bontemps P., Le Fur R., Van Den Bogaert W., Caspers R., Van Glabbeke M. The predictive value of cell kinetic measurements in a European trial of accelerated fractionation in advanced head and neck tumors: an interim report. Int J Radiat Oncol Biol Phys. 1990 Dec;19(6):1449–1453. doi: 10.1016/0360-3016(90)90357-p. [DOI] [PubMed] [Google Scholar]
- Begg A. C., McNally N. J., Shrieve D. C., Kärcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985 Nov;6(6):620–626. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- Begg A. C., Moonen L., Hofland I., Dessing M., Bartelink H. Human tumour cell kinetics using a monoclonal antibody against iododeoxyuridine: intratumour sampling variations. Radiother Oncol. 1988 Apr;11(4):337–347. doi: 10.1016/0167-8140(88)90205-8. [DOI] [PubMed] [Google Scholar]
- Dische S., Saunders M. I. Continuous, hyperfractionated, accelerated radiotherapy (CHART). Br J Cancer. 1989 Mar;59(3):325–326. doi: 10.1038/bjc.1989.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewinko B., Yang L. Y., Barlogie B., Trujillo J. M. Cultured human tumour cells may be arrested in all stages of the cycle during stationary phase: demonstration of quiescent cells in G1, S and G2 phase. Cell Tissue Kinet. 1984 Sep;17(5):453–463. doi: 10.1111/j.1365-2184.1984.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Rew D. A., Campbell I. D., Taylor I., Wilson G. D. Proliferation indices of invasive breast carcinomas after in vivo 5-bromo-2'-deoxyuridine labelling: a flow cytometric study of 75 tumours. Br J Surg. 1992 Apr;79(4):335–339. doi: 10.1002/bjs.1800790418. [DOI] [PubMed] [Google Scholar]
- Rew D. A., Wilson G. D., Taylor I., Weaver P. C. Proliferation characteristics of human colorectal carcinomas measured in vivo. Br J Surg. 1991 Jan;78(1):60–66. doi: 10.1002/bjs.1800780120. [DOI] [PubMed] [Google Scholar]
- Riccardi A., Giordano M., Danova M., Girino M., Brugnatelli S., Ucci G., Mazzini G. Cell kinetics with in vivo bromodeoxyuridine and flow cytometry: clinical significance in acute non-lymphoblastic leukaemia. Eur J Cancer. 1991;27(7):882–887. doi: 10.1016/0277-5379(91)90139-5. [DOI] [PubMed] [Google Scholar]
- Tubiana M., Courdi A. Cell proliferation kinetics in human solid tumors: relation to probability of metastatic dissemination and long-term survival. Radiother Oncol. 1989 May;15(1):1–18. doi: 10.1016/0167-8140(89)90113-8. [DOI] [PubMed] [Google Scholar]
- Wheeless L. L., Coon J. S., Cox C., Deitch A. D., deVere White R. W., Fradet Y., Koss L. G., Melamed M. R., O'Connell M. J., Reeder J. E. Precision of DNA flow cytometry in inter-institutional analyses. Cytometry. 1991;12(5):405–412. doi: 10.1002/cyto.990120505. [DOI] [PubMed] [Google Scholar]
- White R. A. Computing multiple cell kinetic properties from a single time point. J Theor Biol. 1989 Dec 19;141(4):429–446. doi: 10.1016/s0022-5193(89)80229-2. [DOI] [PubMed] [Google Scholar]
- White R. A., Meistrich M. L. A comment on "A method to measure the duration of DNA synthesis and the potential doubling time from a single sample". Cytometry. 1986 Sep;7(5):486–490. doi: 10.1002/cyto.990070516. [DOI] [PubMed] [Google Scholar]
- White R. A., Terry N. H., Meistrich M. L., Calkins D. P. Improved method for computing potential doubling time from flow cytometric data. Cytometry. 1990;11(2):314–317. doi: 10.1002/cyto.990110214. [DOI] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dische S., Saunders M. I., Des Rochers C., Lewis A. A., Bennett M. H. Measurement of cell kinetics in human tumours in vivo using bromodeoxyuridine incorporation and flow cytometry. Br J Cancer. 1988 Oct;58(4):423–431. doi: 10.1038/bjc.1988.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. D., McNally N. J., Dunphy E., Kärcher H., Pfragner R. The labelling index of human and mouse tumours assessed by bromodeoxyuridine staining in vitro and in vivo and flow cytometry. Cytometry. 1985 Nov;6(6):641–647. doi: 10.1002/cyto.990060621. [DOI] [PubMed] [Google Scholar]


