Abstract
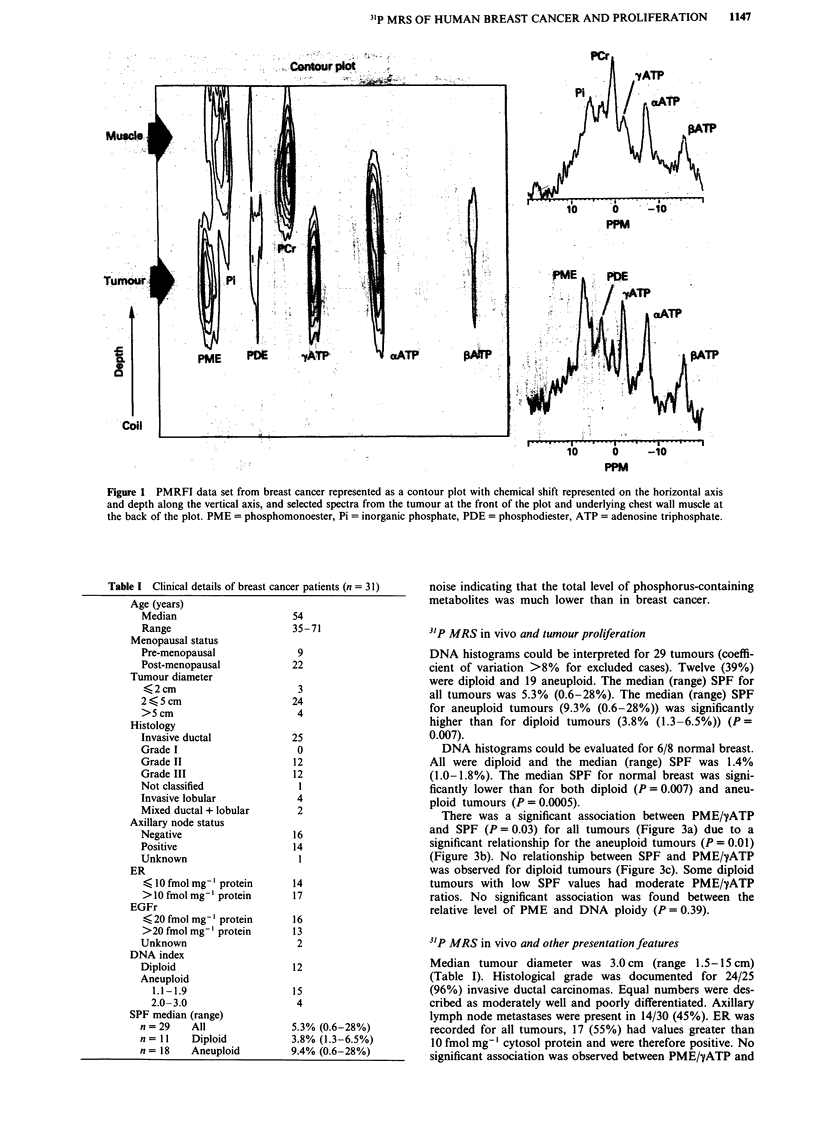
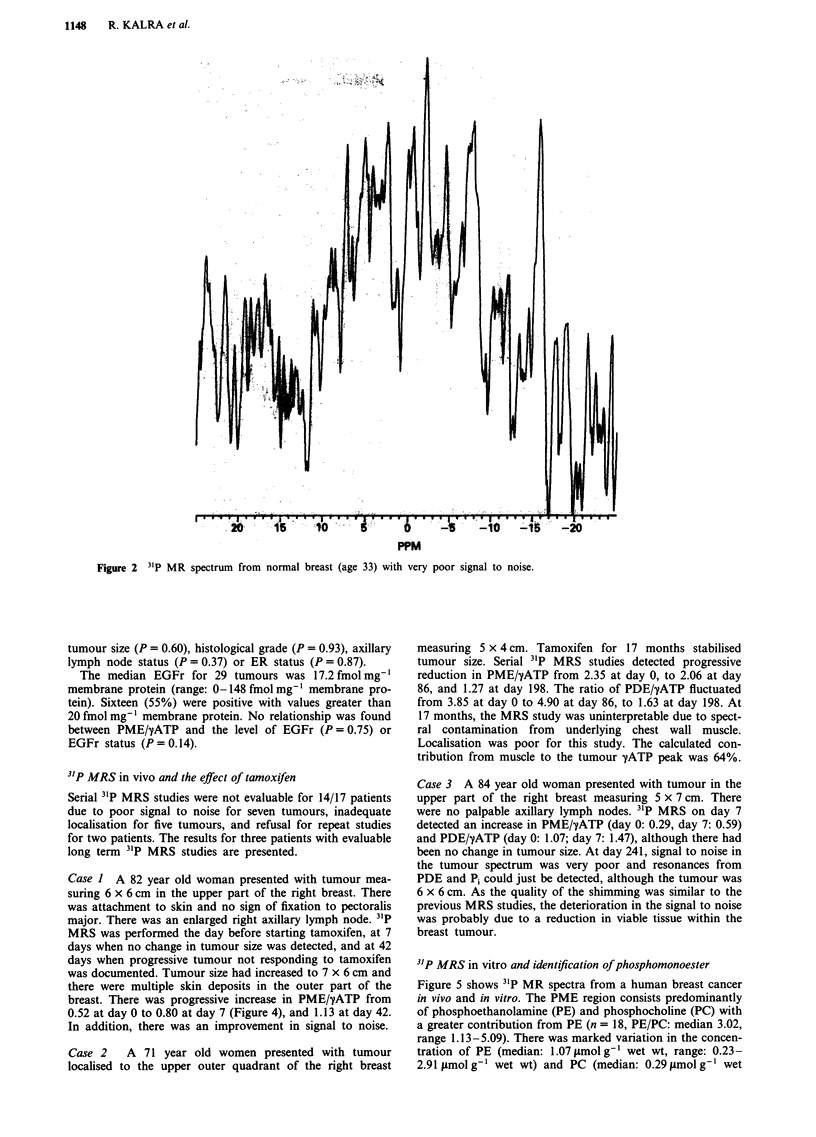
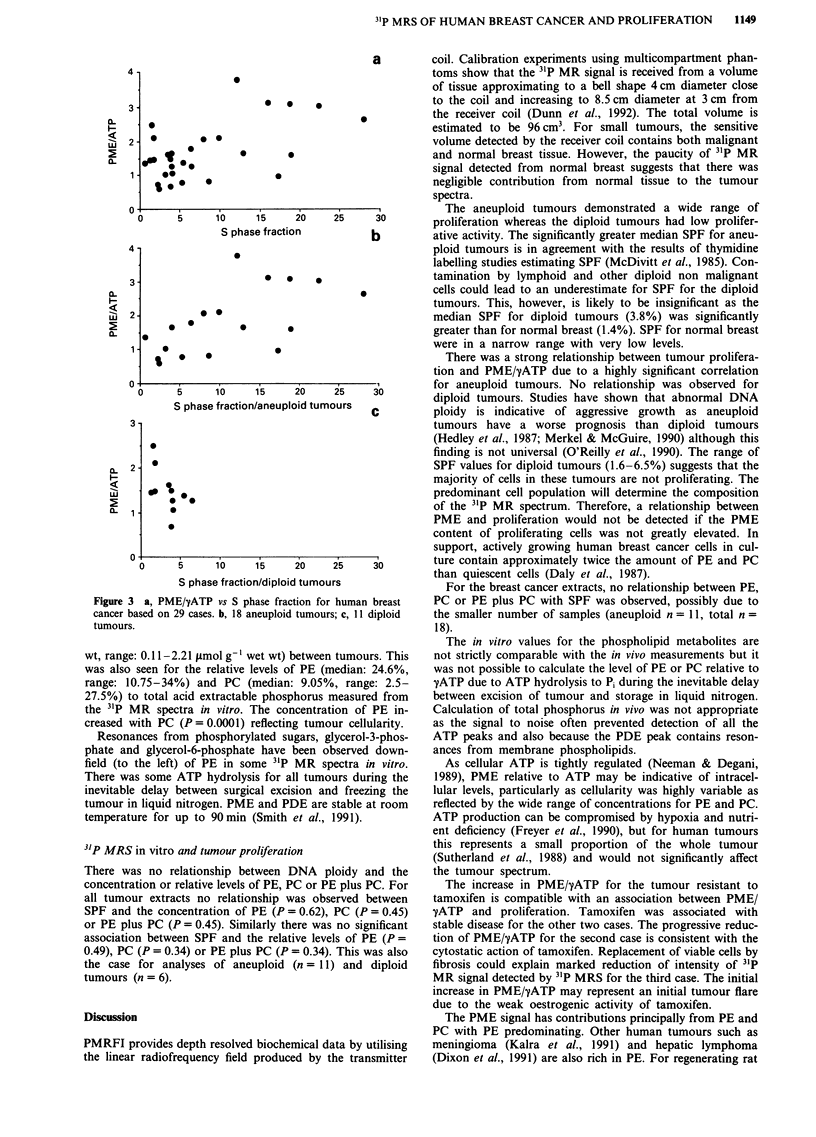
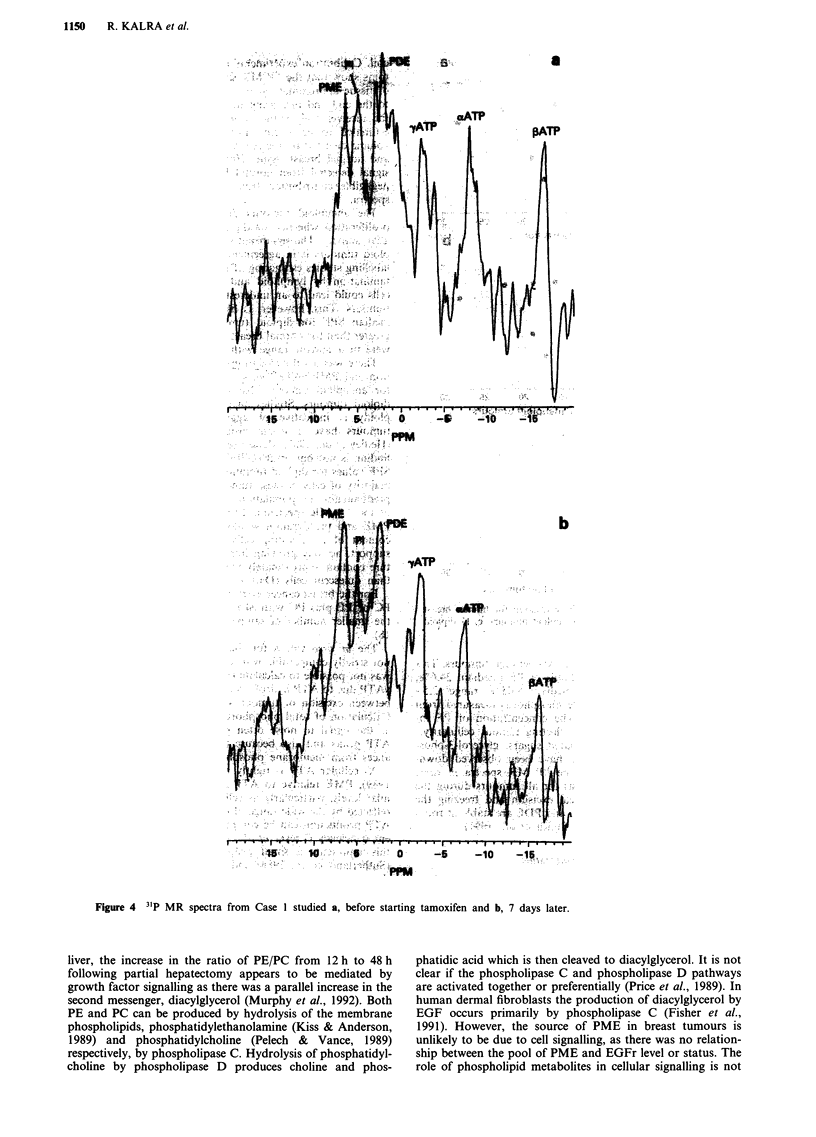
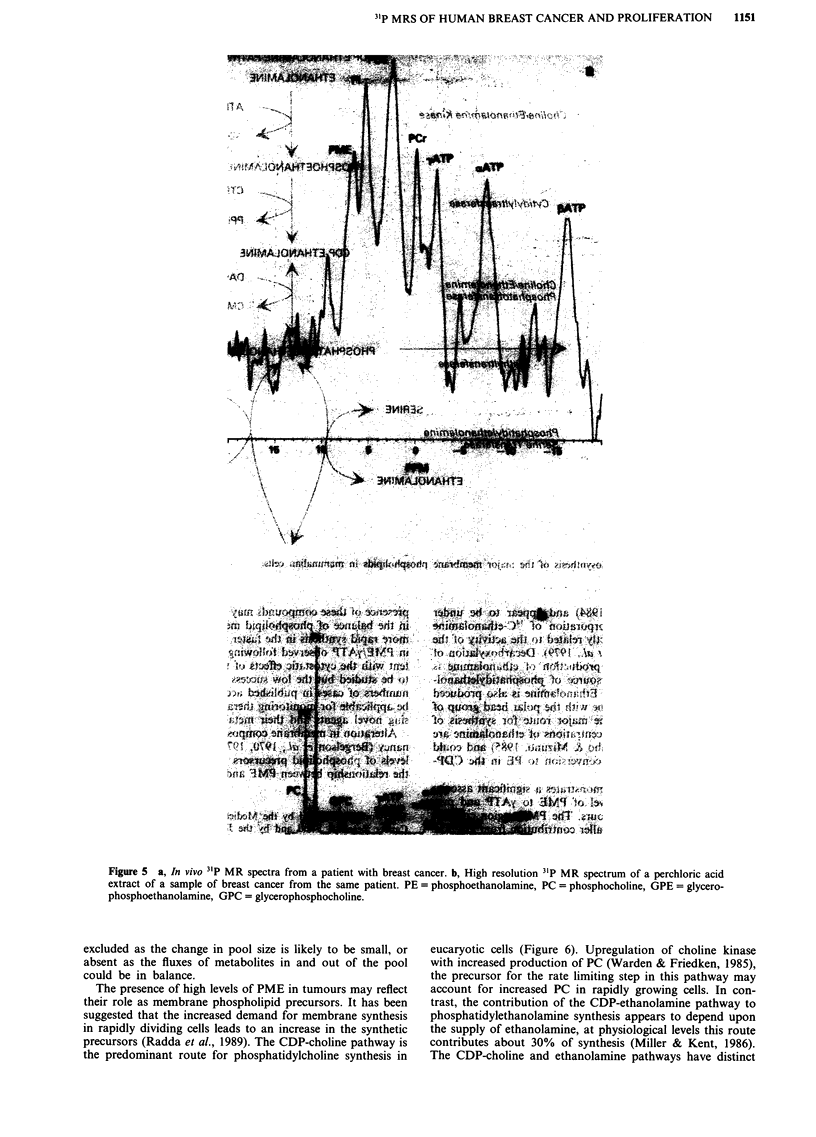
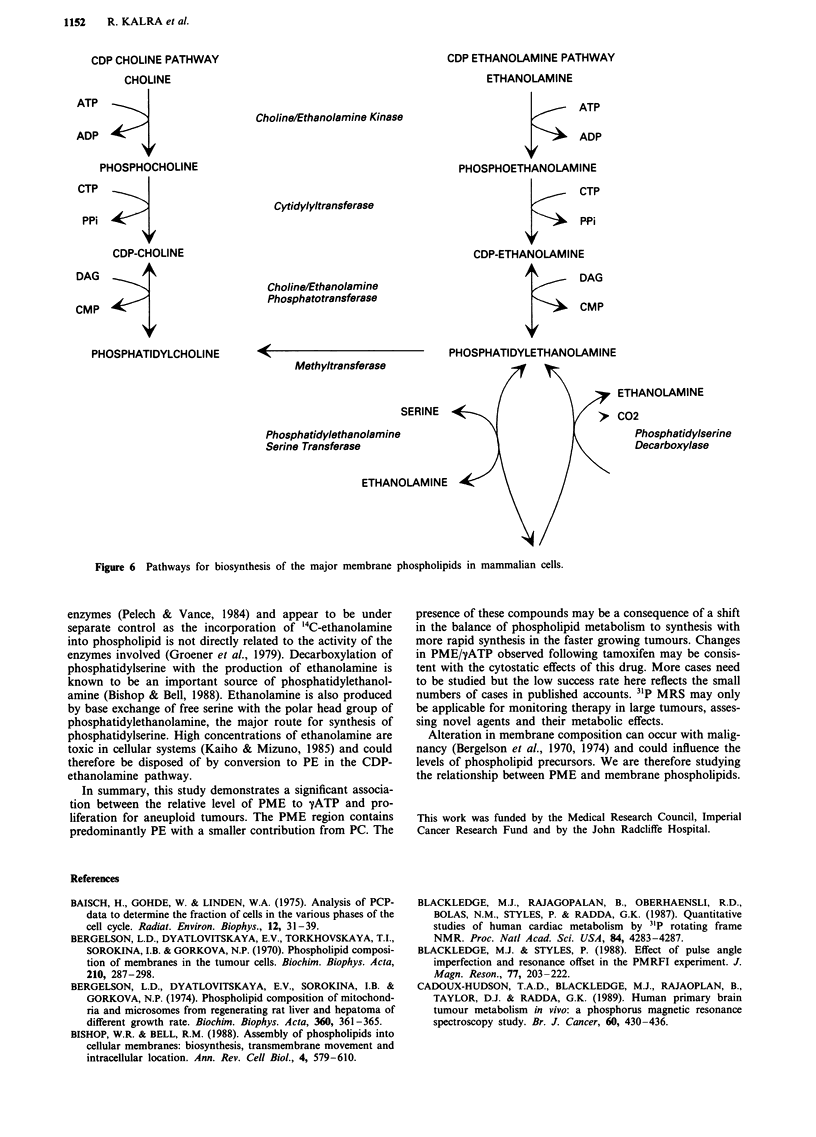
Phospholipid metabolism of human breast cancer was studied by 31P magnetic resonance spectroscopy (MRS). In vivo localised 31P MR spectra were obtained from the tumour alone using phase modulated rotating frame imaging. For 31 tumours, median (range) phosphomonoester (PME) to ATP ratio was 1.48 (0.57-3.78) and phosphodiester (PDE) to ATP ratio was 1.65 (0.44-3.89). DNA index and S phase fraction (SPF) were measured by flow cytometry of paraffin embedded tissue. Twelve (39%) tumours were diploid and 19 aneuploid. Median (range) SPF for 29 assessable tumours was 5.3% (0.6-28%), with significantly greater median SPF for aneuploid tumours (9.3%) than diploid (3.8%, P = 0.007). There was a significant association between PME/ATP and SPF (P = 0.03) due to a significant correlation for aneuploid tumours (P = 0.01). High resolution 31P MRS of extracts from 18 tumours (including seven studied in vivo) demonstrated that the PME peak consists predominantly of phosphoethanolamine (PE) with a smaller contribution from phosphocholine (PC) (median (range) PE/PC: 3.02 (1.13-5.09)). Changes in PME/ATP were observed for two tumours where tamoxifen stablized disease and may be consistent with the cytostatic effects of this drug.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baisch H., Göhde W., Linden W. A. Analysis of PCP-data to determine the fraction of cells in the various phases of cell cycle. Radiat Environ Biophys. 1975 Jun 13;12(1):31–39. doi: 10.1007/BF02339807. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Sorokina I. B., Gorkova N. P. Phospholipid compositon of mitochondria and microsomes from regenerating rat liver and hepatomas of different growth rate. Biochim Biophys Acta. 1974 Sep 19;360(3):361–365. doi: 10.1016/0005-2760(74)90067-8. [DOI] [PubMed] [Google Scholar]
- Bergelson L. D., Dyatlovitskaya E. V., Torkhovskaya T. I., Sorokina I. B., Gorkova N. P. Phospholipid composition of membranes in the tumor cell. Biochim Biophys Acta. 1970 Jul 14;210(2):287–298. doi: 10.1016/0005-2760(70)90173-6. [DOI] [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Assembly of phospholipids into cellular membranes: biosynthesis, transmembrane movement and intracellular translocation. Annu Rev Cell Biol. 1988;4:579–610. doi: 10.1146/annurev.cb.04.110188.003051. [DOI] [PubMed] [Google Scholar]
- Blackledge M. J., Rajagopalan B., Oberhaensli R. D., Bolas N. M., Styles P., Radda G. K. Quantitative studies of human cardiac metabolism by 31P rotating-frame NMR. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4283–4287. doi: 10.1073/pnas.84.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoux-Hudson T. A., Blackledge M. J., Rajagopalan B., Taylor D. J., Radda G. K. Human primary brain tumour metabolism in vivo: a phosphorus magnetic resonance spectroscopy study. Br J Cancer. 1989 Sep;60(3):430–436. doi: 10.1038/bjc.1989.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camplejohn R. S., Macartney J. C., Morris R. W. Measurement of S-phase fractions in lymphoid tissue comparing fresh versus paraffin-embedded tissue and 4',6'-diamidino-2 phenylindole dihydrochloride versus propidium iodide staining. Cytometry. 1989 Jul;10(4):410–416. doi: 10.1002/cyto.990100408. [DOI] [PubMed] [Google Scholar]
- Daly P. F., Lyon R. C., Faustino P. J., Cohen J. S. Phospholipid metabolism in cancer cells monitored by 31P NMR spectroscopy. J Biol Chem. 1987 Nov 5;262(31):14875–14878. [PubMed] [Google Scholar]
- Dixon R. M., Angus P. W., Rajagopalan B., Radda G. K. Abnormal phosphomonoester signals in 31P MR spectra from patients with hepatic lymphoma. A possible marker of liver infiltration and response to chemotherapy. Br J Cancer. 1991 Jun;63(6):953–958. doi: 10.1038/bjc.1991.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. F., Kemp G. J., Radda G. K. Depth selective quantification of phosphorus metabolites in human calf muscle. NMR Biomed. 1992 May-Jun;5(3):154–160. doi: 10.1002/nbm.1940050309. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Sakai T. T., Ng T. C., Krishna N. R., Kim H. D., Zeidler R. B., Ghanta V. K., Brockman R. W., Schiffer L. M., Braunschweiger P. G. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984 Sep 14;805(1):104–116. doi: 10.1016/0167-4889(84)90042-9. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Henderson P. A., Voorhees J. J., Baldassare J. J. Epidermal growth factor-induced hydrolysis of phosphatidylcholine by phospholipase D and phospholipase C in human dermal fibroblasts. J Cell Physiol. 1991 Feb;146(2):309–317. doi: 10.1002/jcp.1041460216. [DOI] [PubMed] [Google Scholar]
- Glaholm J., Leach M. O., Collins D. J., Mansi J., Sharp J. C., Madden A., Smith I. E., McCready V. R. In-vivo 31P magnetic resonance spectroscopy for monitoring treatment response in breast cancer. Lancet. 1989 Jun 10;1(8650):1326–1327. doi: 10.1016/s0140-6736(89)92717-7. [DOI] [PubMed] [Google Scholar]
- Glass A. G., Hoover R. N. Rising incidence of breast cancer: relationship to stage and receptor status. J Natl Cancer Inst. 1990 Apr 18;82(8):693–696. doi: 10.1093/jnci/82.8.693. [DOI] [PubMed] [Google Scholar]
- Groener J. E., Klein W., Van Golde L. M. The effect of fasting and refeeding on the composition and synthesis of triacylglycerols, phosphatidylcholines, and phosphatidylethanolamines in rat liver. Arch Biochem Biophys. 1979 Nov;198(1):287–295. doi: 10.1016/0003-9861(79)90421-1. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Rugg C. A., Gelber R. D. Association of DNA index and S-phase fraction with prognosis of nodes positive early breast cancer. Cancer Res. 1987 Sep 1;47(17):4729–4735. [PubMed] [Google Scholar]
- Hope P. L., Costello A. M., Cady E. B., Delpy D. T., Tofts P. S., Chu A., Hamilton P. A., Reynolds E. O., Wilkie D. R. Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal and birth-asphyxiated infants. Lancet. 1984 Aug 18;2(8399):366–370. doi: 10.1016/s0140-6736(84)90539-7. [DOI] [PubMed] [Google Scholar]
- Kaiho S., Mizuno K. Inhibition of Friend cell erythrodifferentiation by modification of membrane phospholipid composition by choline analogues. Biochim Biophys Acta. 1985 Jan 28;838(1):175–178. doi: 10.1016/0304-4165(85)90264-8. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
- Leake R. E., Laing L., Calman K. C., Macbeth F. R., Crawford D., Smith D. C. Oestrogen-receptor status and endocrine therapy of breast cancer: response rates and status stability. Br J Cancer. 1981 Jan;43(1):59–66. doi: 10.1038/bjc.1981.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDivitt R. W., Stone K. R., Craig R. B., Meyer J. S. A comparison of human breast cancer cell kinetics measured by flow cytometry and thymidine labeling. Lab Invest. 1985 Mar;52(3):287–291. [PubMed] [Google Scholar]
- Merkel D. E., McGuire W. L. Ploidy, proliferative activity and prognosis. DNA flow cytometry of solid tumors. Cancer. 1990 Mar 1;65(5):1194–1205. doi: 10.1002/1097-0142(19900301)65:5<1194::aid-cncr2820650528>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Kent C. Characterization of the pathways for phosphatidylethanolamine biosynthesis in Chinese hamster ovary mutant and parental cell lines. J Biol Chem. 1986 Jul 25;261(21):9753–9761. [PubMed] [Google Scholar]
- Moorcraft J., Bolas N. M., Ives N. K., Ouwerkerk R., Smyth J., Rajagopalan B., Hope P. L., Radda G. K. Global and depth resolved phosphorus magnetic resonance spectroscopy to predict outcome after birth asphyxia. Arch Dis Child. 1991 Oct;66(10 Spec No):1119–1123. doi: 10.1136/adc.66.10_spec_no.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman M., Degani H. Early estrogen-induced metabolic changes and their inhibition by actinomycin D and cycloheximide in human breast cancer cells: 31P and 13C NMR studies. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5585–5589. doi: 10.1073/pnas.86.14.5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng T. C., Grundfest S., Vijayakumar S., Baldwin N. J., Majors A. W., Karalis I., Meaney T. F., Shin K. H., Thomas F. J., Tubbs R. Therapeutic response of breast carcinoma monitored by 31P MRS in situ. Magn Reson Med. 1989 Apr;10(1):125–134. doi: 10.1002/mrm.1910100112. [DOI] [PubMed] [Google Scholar]
- Nicholson S., Sainsbury J. R., Needham G. K., Chambers P., Farndon J. R., Harris A. L. Quantitative assays of epidermal growth factor receptor in human breast cancer: cut-off points of clinical relevance. Int J Cancer. 1988 Jul 15;42(1):36–41. doi: 10.1002/ijc.2910420108. [DOI] [PubMed] [Google Scholar]
- O'Reilly S. M., Camplejohn R. S., Barnes D. M., Millis R. R., Allen D., Rubens R. D., Richards M. A. DNA index, S-phase fraction, histological grade and prognosis in breast cancer. Br J Cancer. 1990 May;61(5):671–674. doi: 10.1038/bjc.1990.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhaensli R. D., Hilton-Jones D., Bore P. J., Hands L. J., Rampling R. P., Radda G. K. Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet. 1986 Jul 5;2(8497):8–11. doi: 10.1016/s0140-6736(86)92558-4. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Vance D. E. Regulation of phosphatidylcholine biosynthesis. Biochim Biophys Acta. 1984 Jun 25;779(2):217–251. doi: 10.1016/0304-4157(84)90010-8. [DOI] [PubMed] [Google Scholar]
- Price B. D., Morris J. D., Hall A. Stimulation of phosphatidylcholine breakdown and diacylglycerol production by growth factors in Swiss-3T3 cells. Biochem J. 1989 Dec 1;264(2):509–515. doi: 10.1042/bj2640509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radda G. K., Rajagopalan B., Taylor D. J. Biochemistry in vivo: an appraisal of clinical magnetic resonance spectroscopy. Magn Reson Q. 1989 Apr;5(2):122–151. [PubMed] [Google Scholar]
- Shapiro S., Venet W., Strax P., Venet L., Roeser R. Ten- to fourteen-year effect of screening on breast cancer mortality. J Natl Cancer Inst. 1982 Aug;69(2):349–355. [PubMed] [Google Scholar]
- Styles P. Passive electrical isolation of double coil probes for localized spectroscopy and imaging. NMR Biomed. 1988 Apr;1(2):61–66. doi: 10.1002/nbm.1940010202. [DOI] [PubMed] [Google Scholar]
- Styles P. Quantitative spectroscopy using multiple surface coil probes. Magn Reson Med. 1991 Jan;17(1):3–9. doi: 10.1002/mrm.1910170103. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Rasey J. S., Hill R. P. Tumor biology. Am J Clin Oncol. 1988 Jun;11(3):253–274. doi: 10.1097/00000421-198806000-00004. [DOI] [PubMed] [Google Scholar]
- Tabár L., Fagerberg C. J., Gad A., Baldetorp L., Holmberg L. H., Gröntoft O., Ljungquist U., Lundström B., Månson J. C., Eklund G. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet. 1985 Apr 13;1(8433):829–832. doi: 10.1016/s0140-6736(85)92204-4. [DOI] [PubMed] [Google Scholar]
- Warden C. H., Friedkin M. Regulation of choline kinase activity and phosphatidylcholine biosynthesis by mitogenic growth factors in 3T3 fibroblasts. J Biol Chem. 1985 May 25;260(10):6006–6011. [PubMed] [Google Scholar]
- Younkin D. P., Delivoria-Papadopoulos M., Leonard J. C., Subramanian V. H., Eleff S., Leigh J. S., Jr, Chance B. Unique aspects of human newborn cerebral metabolism evaluated with phosphorus nuclear magnetic resonance spectroscopy. Ann Neurol. 1984 Nov;16(5):581–586. doi: 10.1002/ana.410160509. [DOI] [PubMed] [Google Scholar]


