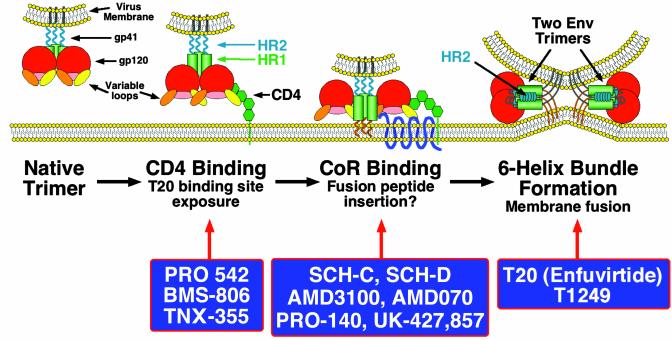
Fig. 1.
A model for HIV entry is shown, with the steps prevented by different entry inhibitors shown rather than the step at which each entry inhibitor binds. For example, T20 binds to Env after it engages CD4 (second section), but it blocks six-helix bundle formation (fourth section). BMS-806 binds to the native Env (first section) and prevents binding to CD4 (second section).The Env protein is a homotrimeric protein with each subunit containing surface gp120 and membrane-spanning gp41 proteins. The native Env trimer is a target for neutralizing antibodies (first section), although few other broadly cross-reactive, neutralizing antibodies have been described. Binding to CD4 is mediated by the gp120 subunit, and this can be inhibited by the small molecule inhibitor BMS-806 (Bristol-Myers Squibb) by the CD4-IgG2 chimera PRO 542 (Progenics, Tarrytown, NY) and by the anti-CD4 antibody TNX-355 (Tanox, Houston) (second section). CD4 binding induces conformational changes in gp120 that result in the exposure of a conserved region that participates in coreceptor binding (second section). This conserved region is, in the native trimer, hidden in part by variable loops that are thought to be repositioned after CD4 binding. Although only a single CD4-binding event is shown, multiple CD4-binding events may be needed to activate a single Env trimer. CD4 binding also makes Env a target for the fusion inhibitor T20, a peptide that binds to a triple-stranded coiled coil in the N-terminal region of gp41 that is formed by the helical HR1 domains (shown by green cylinders). A more potent fusion inhibitor that has completed early clinical trials, T1249, also targets this region. It is not known with what efficiency the T20-binding site is exposed by CD4 binding alone, and it is possible that coreceptor binding may be needed to cause full exposure. After CD4 binding, gp120 binds to a seven-transmembrane domain coreceptor (third section, CoR). Coreceptor binding can be inhibited by several CCR5 blockers that are under clinical development including SCH-C and SCH-D (both from Schering-Plough) and UK-427,857 (from Pfizer, Sandwich, U.K.; C. Hitchcock, personal communication), by the anti-CCR5 antibody PRO-140 (Progenics), and by the CXCR4 inhibitors AMD3100 and AMD070 (both from AnorMED, Vancouver). The hydrophobic fusion peptide at the N terminus of gp41 becomes exposed and inserts into the membrane of the cell. Whether this results from CD4 binding or coreceptor binding is not known. Coreceptor binding ultimately results in formation of a six-helix bundle in which the helical HR2 domains in each gp41 subunit fold back and pack into grooves on the outside of the triple-stranded HR1 domains (fourth section), bringing the fusion peptide and transmembrane domain of gp41 (and their associated membranes) into close proximity. It is likely that several Env trimers need to undergo this conformational change in order to form a fusion pore, although here only two trimers are depicted. It is not known whether gp120 remains associated during the fusion process or dissociates from gp41.