Fig. 3.
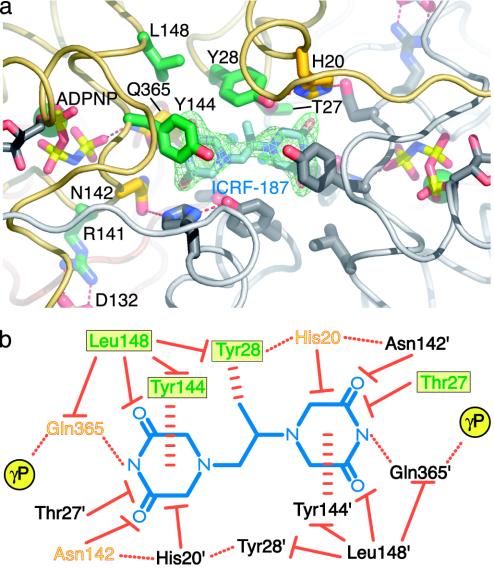
ICRF-187-binding pocket and protein/drug interactions. (a) ICRF-187-binding pocket seen from the top of the dimer. An Fobs–Fcalc simulated-anneal omit electron density map shown in green is contoured at 1.5σ around ICRF-187. ADPNP, ICRF-187, and residues within 5 Å of the drug are shown in stick representation. For one protomer the residue positions correlated with drug resistance are colored dark green and other neighboring amino acids have been colored yellow. Residue side chains from the second protomer are colored gray. (b) Schematic diagram of protein/drug interactions. ICRF-187 is blue. Residues contacting the drug from each of the two protomers are indicated by colored or black text. For the colored protomer, the green residues in a yellow box indicate that drug-resistance mutations have been isolated at these positions. Orange/yellow residues are within 5 Å of ICRF-187 but have not yet been shown to affect drug efficacy when mutated. Hydrogen bonds are indicated by dotted red lines, stacking interactions are indicated by horizontally dashed red lines, and van der Waals interactions are indicated by solid red lines with a flat end. The γ-phosphates of bound ADPNPs are indicated by yellow circles.