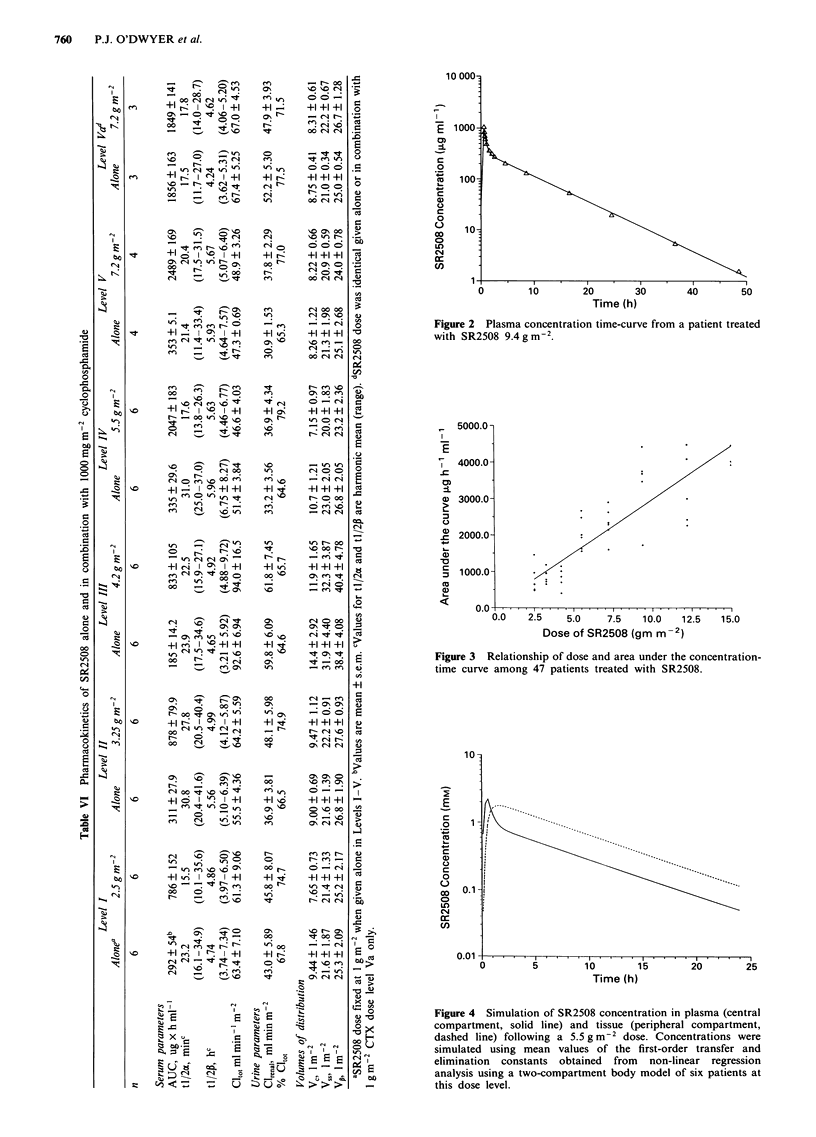
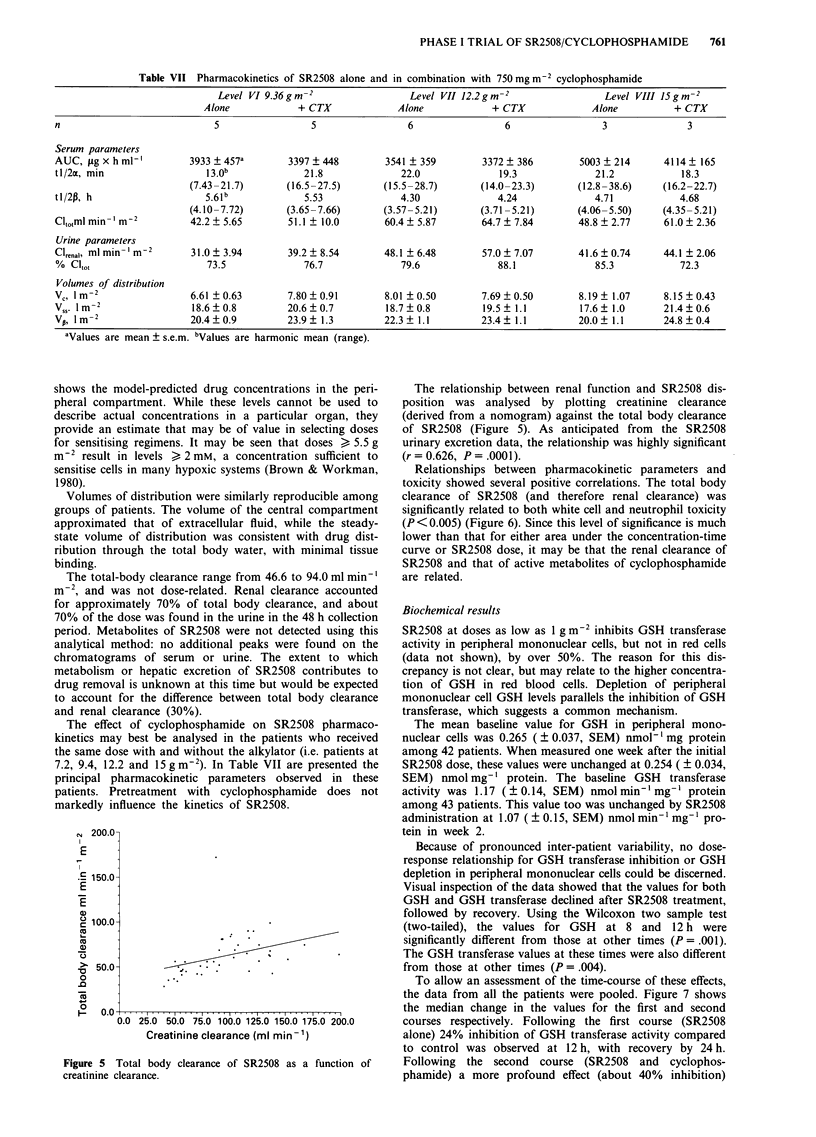
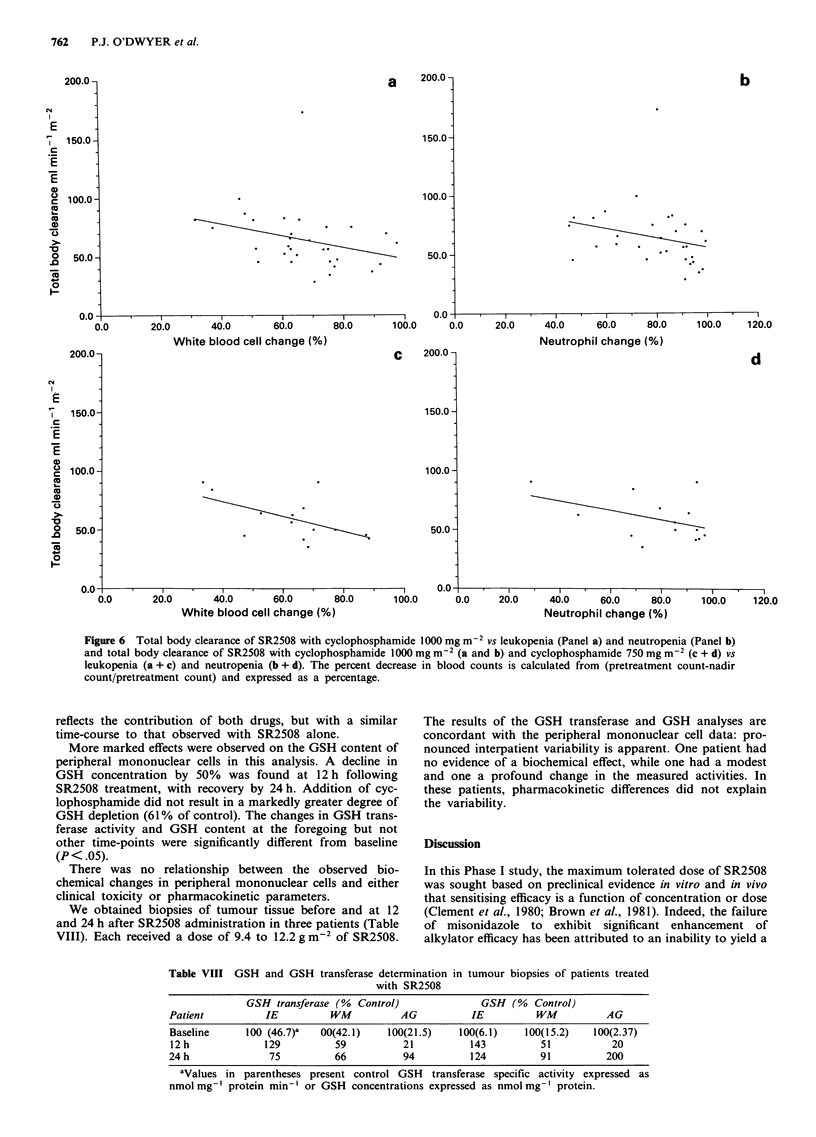
Abstract
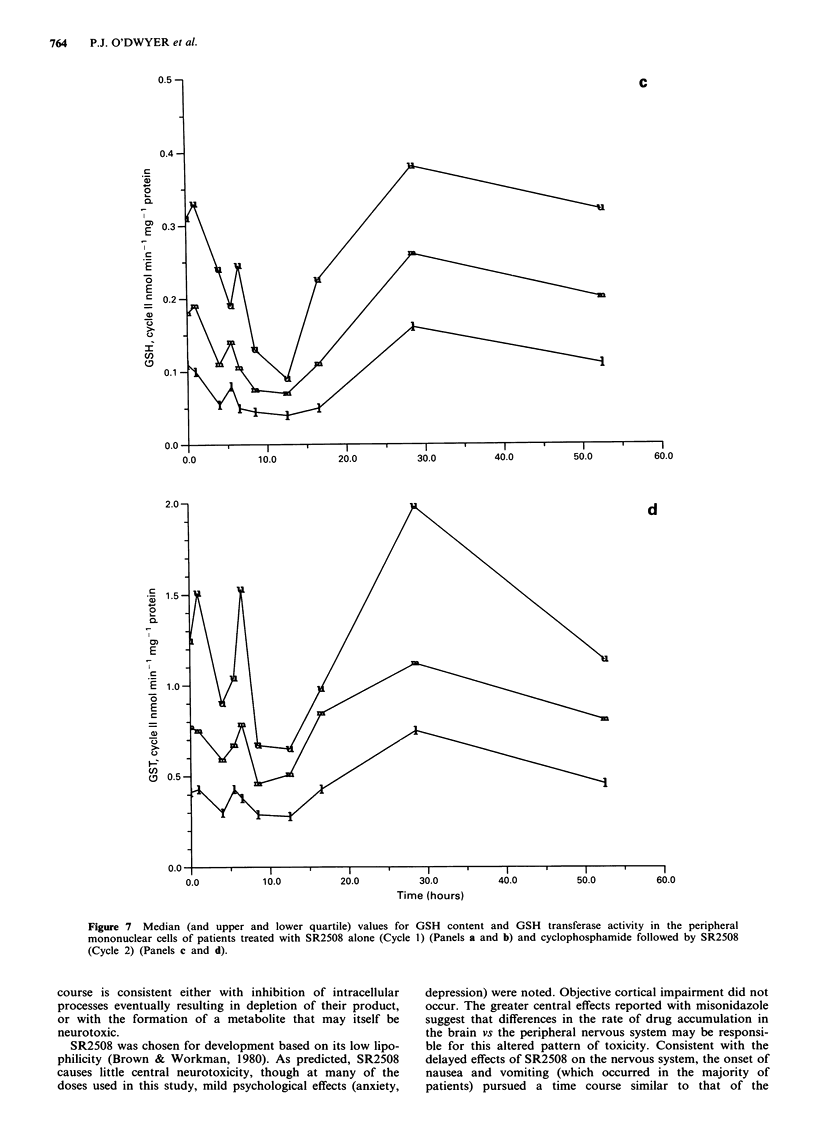
SR2508 sensitises certain hypoxic tumor cells in vitro and in vivo to the cytotoxic action of radiation and alkylating agents. The mechanism of sensitisation may derive in part from depletion of glutathione (GSH) and possibly inhibition of GSH-dependent enzymes in target cells. We treated 46 evaluable patients with cyclophosphamide 750-1000 mg m-2 followed by SR2508 at eight dose levels ranging from 2.5 to 15.0 g m-2. Each patient received SR2508 as a single agent initially, followed a week later by the combination of cyclophosphamide and SR2508. Initially, myelosuppression was the major toxicity; potentiation of cyclophosphamide-induced leukopenia by SR2508 required a dose reduction of cyclophosphamide to 750 mg m-2 at SR2508 doses above 7.2 g m-2. At doses above 9.4 g m-2 an acute syndrome of muscle pains and painful paresthesias of the extremities lasting 12-24 h was observed to occur with increasing severity. This side-effect was intolerable in two of three patients treated at 15.0 g m-2. The only other reproducible side-effect was nausea and vomiting which was controllable with antiemetics. Plasma and urine SR2508 concentrations were measured by HPLC in 45 patients. Plasma elimination curves fit a 2-compartment model. The mean terminal half-life at each dose level ranged from 5.1-5.8 h. The mean area under the plasma concentration-time curve was linearly related to dose, and mean total body clearance ranged from 46.6-94.0 ml-1 min-1 m-2; renal clearance accounted for 65.7-79.3%. Pretreatment with cyclophosphamide did not influence the kinetics of SR2508 in individual patients. Examination of the glutathione content of peripheral mononuclear cells and tumour samples showed that depletion to below 50% of control occurred in the majority of patients. GSH transferase inhibition occurred with a similar time-course, but to a lesser extent. These data suggest that the further evaluation of this regimen should be conducted with SR2508 administration preceding that of cyclophosphamide and that its evaluation in cyclophosphamide-sensitive tumours is warranted.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. E., Stratford I. J. Hypoxia-mediated nitro-heterocyclic drugs in the radio- and chemotherapy of cancer. An overview. Biochem Pharmacol. 1986 Jan 1;35(1):71–76. doi: 10.1016/0006-2952(86)90560-5. [DOI] [PubMed] [Google Scholar]
- Allalunis M. J., Chapman J. D., Turner A. R. Identification of a hypoxic population of bone marrow cells. Int J Radiat Oncol Biol Phys. 1983 Feb;9(2):227–232. doi: 10.1016/0360-3016(83)90104-9. [DOI] [PubMed] [Google Scholar]
- Bailey H., Mulcahy R. T., Tutsch K. D., Rozental J. M., Alberti D., Arzoomanian R. Z., Tombes M. B., Trump D. L., Wilding G. A phase I study of SR-2508 and cyclophosphamide administered by intravenous injection. Cancer Res. 1991 Feb 15;51(4):1099–1104. [PubMed] [Google Scholar]
- Brown J. M., Workman P. Partition coefficient as a guide to the development of radiosensitizers which are less toxic than misonidazole. Radiat Res. 1980 Apr;82(1):171–190. [PubMed] [Google Scholar]
- Brown J. M., Yu N. Y., Brown D. M., Lee W. W. SR-2508: a 2-nitroimidazole amide which should be superior to misonidazole as a radiosensitizer for clinical use. Int J Radiat Oncol Biol Phys. 1981 Jun;7(6):695–703. doi: 10.1016/0360-3016(81)90460-0. [DOI] [PubMed] [Google Scholar]
- Clement J. J., Gorman M. S., Wodinsky I., Catane R., Johnson R. K. Enhancement of antitumor activity of alkylating agents by the radiation sensitizer misonidazole. Cancer Res. 1980 Nov;40(11):4165–4172. [PubMed] [Google Scholar]
- Coleman C. N., Bump E. A., Kramer R. A. Chemical modifiers of cancer treatment. J Clin Oncol. 1988 Apr;6(4):709–733. doi: 10.1200/JCO.1988.6.4.709. [DOI] [PubMed] [Google Scholar]
- Coleman C. N., Halsey J., Cox R. S., Hirst V. K., Blaschke T., Howes A. E., Wasserman T. H., Urtasun R. C., Pajak T., Hancock S. Relationship between the neurotoxicity of the hypoxic cell radiosensitizer SR 2508 and the pharmacokinetic profile. Cancer Res. 1987 Jan 1;47(1):319–322. [PubMed] [Google Scholar]
- Coleman C. N., Urtasun R. C., Wasserman T. H., Hancock S., Harris J. W., Halsey J., Hirst V. K. Initial report of the phase I trial of the hypoxic cell radiosensitizer SR-2508. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1749–1753. doi: 10.1016/0360-3016(84)90542-x. [DOI] [PubMed] [Google Scholar]
- Coleman C. N., Wasserman T. H., Urtasun R. C., Halsey J., Hirst V. K., Hancock S., Phillips T. L. Phase I trial of the hypoxic cell radiosensitizer SR-2508: the results of the five to six week drug schedule. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1105–1108. doi: 10.1016/0360-3016(86)90236-1. [DOI] [PubMed] [Google Scholar]
- Durand R. E., Chaplin D. J. Chemosensitization by misonidazole in CCNU-treated spheroids and tumours. Br J Cancer. 1987 Aug;56(2):103–109. doi: 10.1038/bjc.1987.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grau C., Bentzen S. M., Overgaard J. Cytotoxic effect of misonidazole and cyclophosphamide on aerobic and hypoxic cells in a C3H mammary carcinoma in vivo. Br J Cancer. 1990 Jan;61(1):61–64. doi: 10.1038/bjc.1990.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980 Jul 15;106(1):207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hinchliffe M., McNally N. J., Stratford M. R. The effect of radiosensitizers on the pharmacokinetics of melphalan and cyclophosphamide in the mouse. Br J Cancer. 1983 Sep;48(3):375–383. doi: 10.1038/bjc.1983.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst D. G., Hazlehurst J. L., Brown J. M. Sensitization of normal and malignant tissue to cyclophosphamide by nitroimidazoles with different partition coefficients. Br J Cancer. 1984 Jan;49(1):33–42. doi: 10.1038/bjc.1984.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsman M. R., Wood P. J., Brown J. M. Misonidazole chemosensitization of EMT6 spheroids to melphalan. Radiother Oncol. 1989 May;15(1):103–114. doi: 10.1016/0167-8140(89)90123-0. [DOI] [PubMed] [Google Scholar]
- Kumar K. S., Weiss J. F. Inhibition of glutathione peroxidase and glutathione transferase in mouse liver by misonidazole. Biochem Pharmacol. 1986 Sep 15;35(18):3143–3146. doi: 10.1016/0006-2952(86)90399-0. [DOI] [PubMed] [Google Scholar]
- Law M. P., Hirst D. G., Brown J. M. Enhancing effect of misonidazole on the response of the RIF-1 tumour to cyclophosphamide. Br J Cancer. 1981 Aug;44(2):208–218. doi: 10.1038/bjc.1981.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mulcahy R. T. Cross-link formation and chemopotentiation of EMT-6/Ro cells exposed to MISO after CCNU treatment in vitro. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1389–1392. doi: 10.1016/0360-3016(86)90178-1. [DOI] [PubMed] [Google Scholar]
- Murray D., Meyn R. E. Enhancement of the DNA cross-linking activity of nitrogen mustard by misonidazole and diethyl maleate in a mouse fibrosarcoma tumor in vivo. Cancer Res. 1984 Jan;44(1):91–96. [PubMed] [Google Scholar]
- Ozols R. F., O'Dwyer P. J., Hamilton T. C., Young R. C. The role of glutathione in drug resistance. Cancer Treat Rev. 1990 Dec;17 (Suppl A):45–50. doi: 10.1016/0305-7372(90)90015-8. [DOI] [PubMed] [Google Scholar]
- Rockwell S., Moulder J. E. Hypoxic fractions of human tumors xenografted into mice: a review. Int J Radiat Oncol Biol Phys. 1990 Jul;19(1):197–202. doi: 10.1016/0360-3016(90)90154-c. [DOI] [PubMed] [Google Scholar]
- Roizin-Towle L., Biaglow J. E., Meltzer H. L., Varnes M. E. Factors associated with the preincubation effect of hypoxic cell sensitizers in vitro and their possible implications in chemosensitization. Radiat Res. 1984 Jun;98(3):506–518. [PubMed] [Google Scholar]
- Siemann D. W., Mulcahy R. T. Sensitization of cancer chemotherapeutic agents by nitroheterocyclics. Biochem Pharmacol. 1986 Jan 1;35(1):111–115. doi: 10.1016/0006-2952(86)90567-8. [DOI] [PubMed] [Google Scholar]
- Smith B. R. Hypoxia-enhanced reduction and covalent binding of [2-3H]misonidazole in the perfused rat liver. Biochem Pharmacol. 1984 Apr 15;33(8):1379–1381. doi: 10.1016/0006-2952(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- THOMLINSON R. H., GRAY L. H. The histological structure of some human lung cancers and the possible implications for radiotherapy. Br J Cancer. 1955 Dec;9(4):539–549. doi: 10.1038/bjc.1955.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Y. C., Evans J. W., Brown J. M. Mechanism of sensitization of Chinese hamster ovary cells to melphalan by hypoxic treatment with misonidazole. Cancer Res. 1983 Jul;43(7):3175–3181. [PubMed] [Google Scholar]
- Taylor Y. C., Sawyer J. M., Hsu B., Brown J. M. Mechanism of melphalan crosslink enhancement by misonidazole pretreatment. Int J Radiat Oncol Biol Phys. 1984 Sep;10(9):1603–1607. doi: 10.1016/0360-3016(84)90511-x. [DOI] [PubMed] [Google Scholar]
- Varghese A. J., Whitmore G. F. Identification of a reactive glutathione conjugate as a metabolite of SR-2508 in CHO cells. Int J Radiat Oncol Biol Phys. 1986 Jul;12(7):1223–1226. doi: 10.1016/0360-3016(86)90263-4. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Tew K. D. Increased glutathione-S-transferase activity in a cell line with acquired resistance to nitrogen mustards. Cancer Treat Rep. 1985 Jun;69(6):677–682. [PubMed] [Google Scholar]


