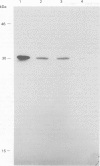
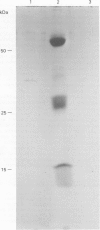
Abstract
The murine monoclonal antibody H17E2 recognises placental alkaline phosphatase (PLAP), an antigen present in the human term placenta and also expressed by many tumours. The antibody is of value in both immunoscintigraphy and radioimmunotherapy in testicular and ovarian cancer. The small size of genetically engineered single chain antibodies (SCAs) should give diagnostic and therapeutic advantages of improved tumour penetration and increased blood clearance compared to IgG. Employing recombinant DNA techniques a SCA based on H17E2 has been expressed in Escherichia coli and has been shown to bind placental alkaline phosphatase specifically. When administered to nude mice bearing human tumour xenografts, the H17E2 SCA effectively localised to tumour whilst a co-administered non-specific SCA did not. H17E2 SCA achieves tumour: blood ratios that are superior to those achieved with whole IgG, probably owing to its rapid blood clearance. We conclude that the H17E2 SCA is suitable for further investigation as an agent for clinical imaging and therapy. Additionally, the SCA can also be used for the construction of antibody based fusion proteins to target other effector functions to tumour cells.
Full text
PDF

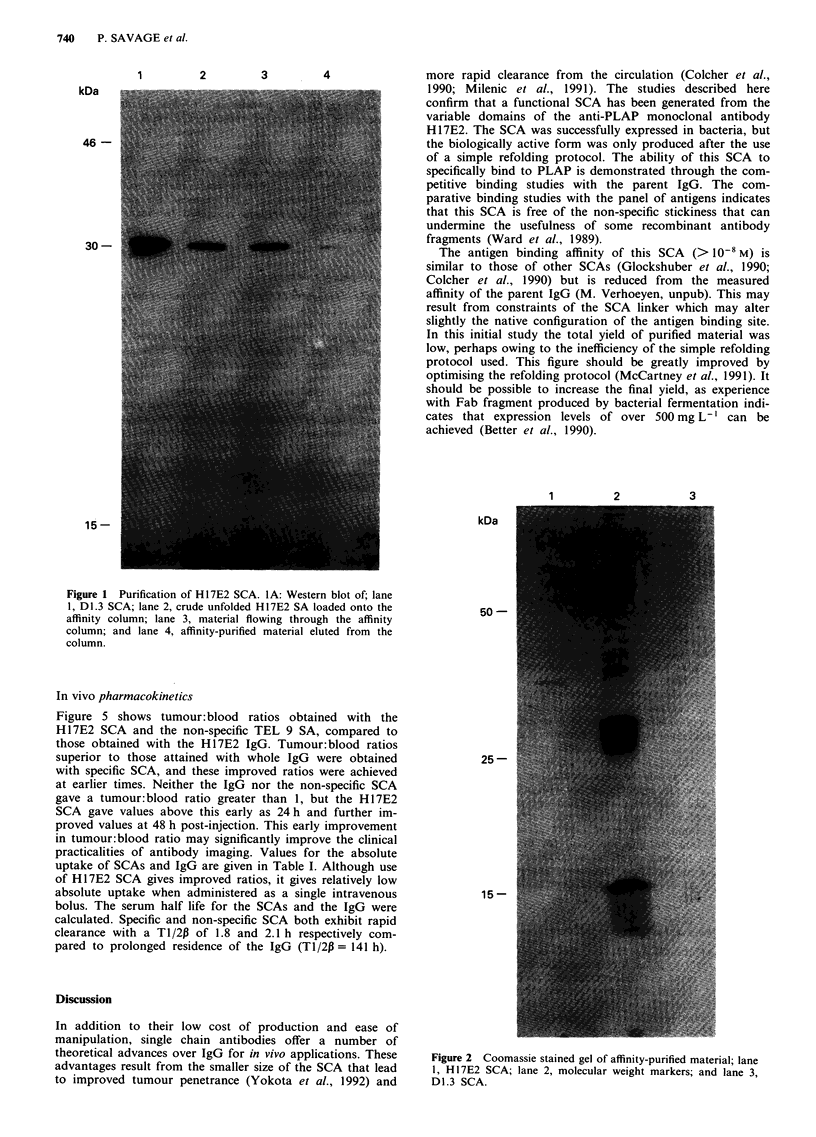
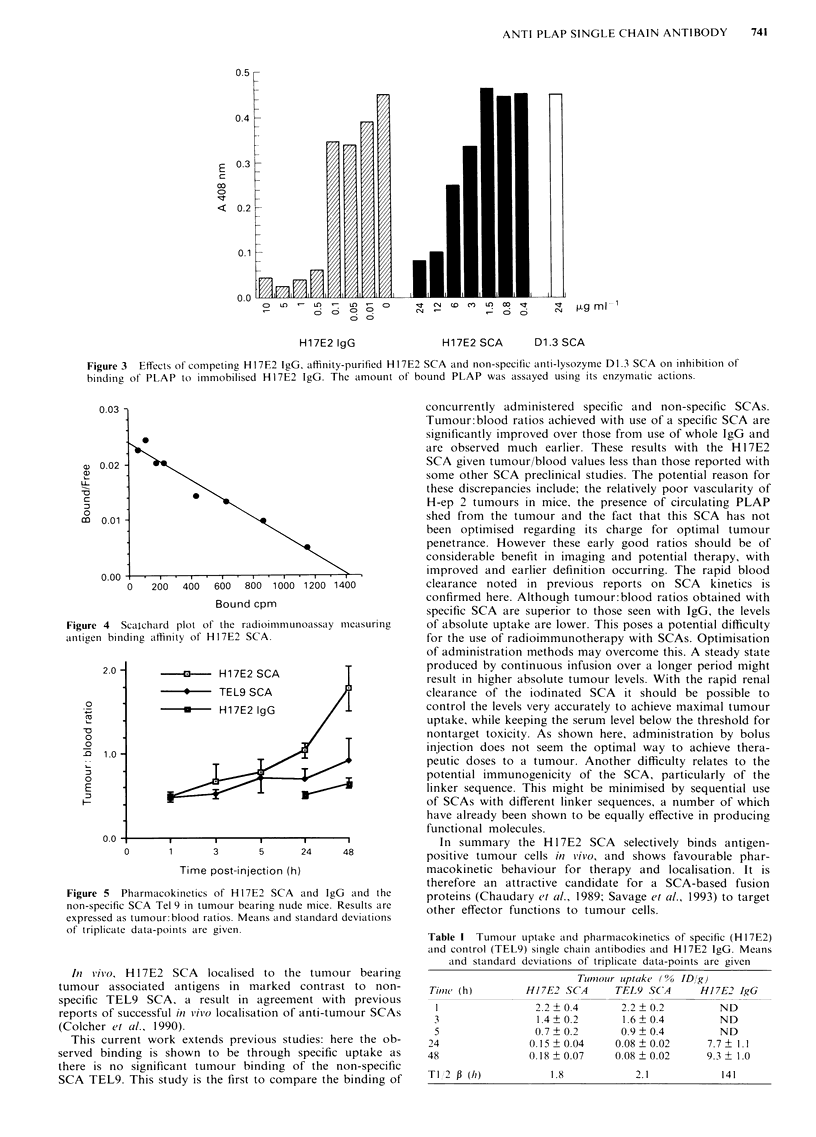

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chaudhary V. K., Queen C., Junghans R. P., Waldmann T. A., FitzGerald D. J., Pastan I. A recombinant immunotoxin consisting of two antibody variable domains fused to Pseudomonas exotoxin. Nature. 1989 Jun 1;339(6223):394–397. doi: 10.1038/339394a0. [DOI] [PubMed] [Google Scholar]
- Colcher D., Bird R., Roselli M., Hardman K. D., Johnson S., Pope S., Dodd S. W., Pantoliano M. W., Milenic D. E., Schlom J. In vivo tumor targeting of a recombinant single-chain antigen-binding protein. J Natl Cancer Inst. 1990 Jul 18;82(14):1191–1197. doi: 10.1093/jnci/82.14.1191. [DOI] [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Moore R., Larche M., Pectasides D., Dhokia B., Ritter M. A. Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res. 1986 Dec;46(12 Pt 1):6489–6493. [PubMed] [Google Scholar]
- Epenetos A. A., Snook D., Durbin H., Johnson P. M., Taylor-Papadimitriou J. Limitations of radiolabeled monoclonal antibodies for localization of human neoplasms. Cancer Res. 1986 Jun;46(6):3183–3191. [PubMed] [Google Scholar]
- Epenetos A. A., Travers P., Gatter K. C., Oliver R. D., Mason D. Y., Bodmer W. F. An immunohistological study of testicular germ cell tumours using two different monoclonal antibodies against placental alkaline phosphatase. Br J Cancer. 1984 Jan;49(1):11–15. doi: 10.1038/bjc.1984.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Glockshuber R., Malia M., Pfitzinger I., Plückthun A. A comparison of strategies to stabilize immunoglobulin Fv-fragments. Biochemistry. 1990 Feb 13;29(6):1362–1367. doi: 10.1021/bi00458a002. [DOI] [PubMed] [Google Scholar]
- Hird V., Verhoeyen M., Badley R. A., Price D., Snook D., Kosmas C., Gooden C., Bamias A., Meares C., Lavender J. P. Tumour localisation with a radioactively labelled reshaped human monoclonal antibody. Br J Cancer. 1991 Nov;64(5):911–914. doi: 10.1038/bjc.1991.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston J. S., Levinson D., Mudgett-Hunter M., Tai M. S., Novotný J., Margolies M. N., Ridge R. J., Bruccoleri R. E., Haber E., Crea R. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5879–5883. doi: 10.1073/pnas.85.16.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennel S. J., Falcioni R., Wesley J. W. Microdistribution of specific rat monoclonal antibodies to mouse tissues and human tumor xenografts. Cancer Res. 1991 Mar 1;51(5):1529–1536. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maraveyas A., Epenetos A. A. An overview of radioimmunotherapy. Cancer Immunol Immunother. 1991;34(2):71–73. doi: 10.1007/BF01741338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks J. D., Hoogenboom H. R., Bonnert T. P., McCafferty J., Griffiths A. D., Winter G. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991 Dec 5;222(3):581–597. doi: 10.1016/0022-2836(91)90498-u. [DOI] [PubMed] [Google Scholar]
- McCafferty J., Griffiths A. D., Winter G., Chiswell D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990 Dec 6;348(6301):552–554. doi: 10.1038/348552a0. [DOI] [PubMed] [Google Scholar]
- McCartney J. E., Lederman L., Drier E. A., Cabral-Denison N. A., Wu G. M., Batorsky R. S., Huston J. S., Oppermann H. Biosynthetic antibody binding sites: development of a single-chain Fv model based on antidinitrophenol IgA myeloma MOPC 315. J Protein Chem. 1991 Dec;10(6):669–683. doi: 10.1007/BF01025718. [DOI] [PubMed] [Google Scholar]
- Milenic D. E., Esteban J. M., Colcher D. Comparison of methods for the generation of immunoreactive fragments of a monoclonal antibody (B72.3) reactive with human carcinomas. J Immunol Methods. 1989 Jun 2;120(1):71–83. doi: 10.1016/0022-1759(89)90291-3. [DOI] [PubMed] [Google Scholar]
- Milenic D. E., Yokota T., Filpula D. R., Finkelman M. A., Dodd S. W., Wood J. F., Whitlow M., Snoy P., Schlom J. Construction, binding properties, metabolism, and tumor targeting of a single-chain Fv derived from the pancarcinoma monoclonal antibody CC49. Cancer Res. 1991 Dec 1;51(23 Pt 1):6363–6371. [PubMed] [Google Scholar]
- Orlandi R., Güssow D. H., Jones P. T., Winter G. Cloning immunoglobulin variable domains for expression by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 May;86(10):3833–3837. doi: 10.1073/pnas.86.10.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage P., So A., Spooner R. A., Epenetos A. A. A recombinant single chain antibody interleukin-2 fusion protein. Br J Cancer. 1993 Feb;67(2):304–310. doi: 10.1038/bjc.1993.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Stewart J. S., Hird V., Snook D., Sullivan M., Hooker G., Courtenay-Luck N., Sivolapenko G., Griffiths M., Myers M. J., Lambert H. E. Intraperitoneal radioimmunotherapy for ovarian cancer: pharmacokinetics, toxicity, and efficacy of I-131 labeled monoclonal antibodies. Int J Radiat Oncol Biol Phys. 1989 Feb;16(2):405–413. doi: 10.1016/0360-3016(89)90337-4. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Johnson K., Beckwith J. Characterization of degP, a gene required for proteolysis in the cell envelope and essential for growth of Escherichia coli at high temperature. J Bacteriol. 1989 May;171(5):2689–2696. doi: 10.1128/jb.171.5.2689-2696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOOLAN H. W. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954 Oct;14(9):660–666. [PubMed] [Google Scholar]
- Travers P., Bodmer W. Preparation and characterization of monoclonal antibodies against placental alkaline phosphatase and other human trophoblast-associated determinants. Int J Cancer. 1984 May 15;33(5):633–641. doi: 10.1002/ijc.2910330514. [DOI] [PubMed] [Google Scholar]
- Vaughan A. T., Anderson P., Dykes P. W., Chapman C. E., Bradwell A. R. Limitations to the killing of tumours using radiolabelled antibodies. Br J Radiol. 1987 Jun;60(714):567–572. doi: 10.1259/0007-1285-60-714-567. [DOI] [PubMed] [Google Scholar]
- Ward E. S., Güssow D., Griffiths A. D., Jones P. T., Winter G. Binding activities of a repertoire of single immunoglobulin variable domains secreted from Escherichia coli. Nature. 1989 Oct 12;341(6242):544–546. doi: 10.1038/341544a0. [DOI] [PubMed] [Google Scholar]
- Yokota T., Milenic D. E., Whitlow M., Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992 Jun 15;52(12):3402–3408. [PubMed] [Google Scholar]