Abstract
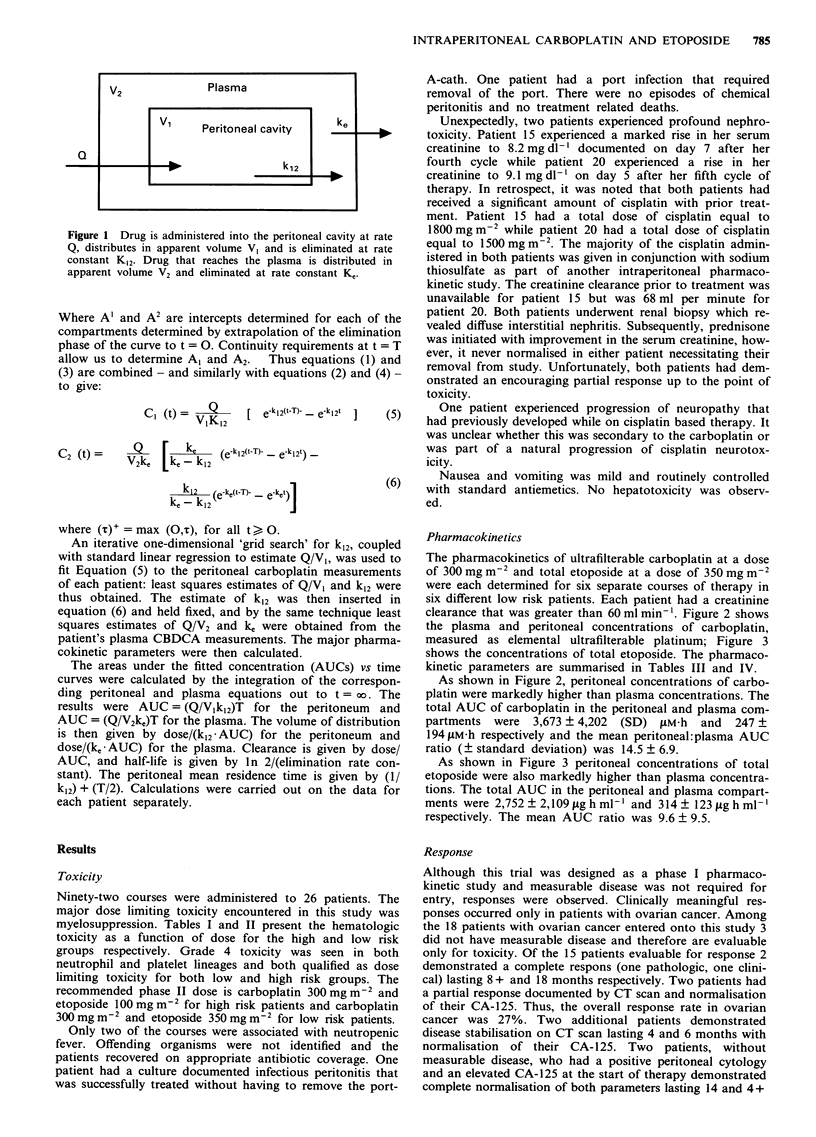
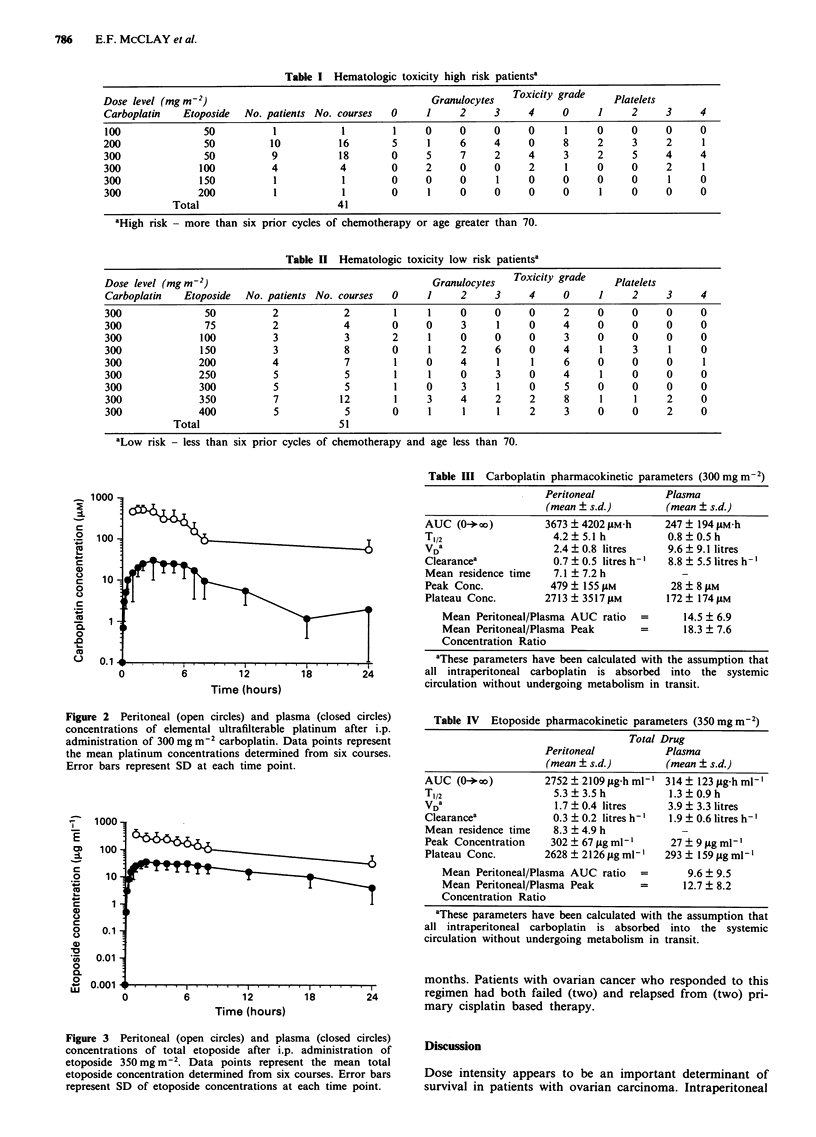
BACKGROUND: We attempted to determine the maximum tolerated dose and toxicity of etoposide (VP-16) when administered in combination with carboplatin (CBDCA) (300 mg m-2) and administered via the intraperitoneal (IP) route. METHODS AND MATERIALS: A total of 26 patients were treated on this trial. CBDCA was administered at a fixed dose of 300 mg m-2) while VP-16 was started at a dose of 200 mg m-2 and escalated at 50 mg m-2 increments. Both agents were mixed together in 2 litres of 5% Dextrose and administered as quickly as possible into the peritoneal cavity. Pharmacokinetic studies were performed at the maximum tolerated dose (MTD). RESULTS: The MTD for this regimen was CBDCA 300 mg m-2 and VP-16 350 mg m-2. Patients > or = 70 years of age or who had received more than six cycles of previous chemotherapy, tolerated this regimen poorly. The MTD for this group of patients was CBDCA 200 mg m-2 and VP-16 50 mg m-2. Neutropenia was the dose limiting toxicity for both groups. The mean peritoneal/plasma peak ratio was 18.3 for CBDCA and 12.7 for VP-16. The pharmacologic advantage (peritoneal/plasma AUC ratio) was 14.9 for CBDCA and 8.8 for VP-16. Although measurable disease was not a requirement for entrance into this study a response rate of 27% was noted in 15 patients with evaluable disease who had ovarian cancer. CONCLUSIONS: A pharmacologic advantage exists for both CBDCA and VP-16 when administered together via the IP route.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts D., Mason N., Surwit E., Weiner S., Hammond N., Deppe G. Phase I trial of carboplatin-cyclophosphamide and iproplatin-cyclophosphamide in advanced ovarian cancer: a Southwest Oncology Group study. Cancer Treat Rev. 1985 Sep;12 (Suppl A):83–92. doi: 10.1016/0305-7372(85)90023-4. [DOI] [PubMed] [Google Scholar]
- Anderson H., Wagstaff J., Crowther D., Swindell R., Lind M. J., McGregor J., Timms M. S., Brown D., Palmer P. Comparative toxicity of cisplatin, carboplatin (CBDCA) and iproplatin (CHIP) in combination with cyclophosphamide in patients with advanced epithelial ovarian cancer. Eur J Cancer Clin Oncol. 1988 Sep;24(9):1471–1479. doi: 10.1016/0277-5379(88)90338-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. F., Raghavan D., Stuart-Harris R., Morstyn G., Aroney R., Kefford R., Yuen K., Lee J., Gianoutsos P., Olver I. N. Carboplatin (CBDCA, JM-8) and VP-16-213 in previously untreated patients with small-cell lung cancer. J Clin Oncol. 1987 Oct;5(10):1574–1578. doi: 10.1200/JCO.1987.5.10.1574. [DOI] [PubMed] [Google Scholar]
- Colombo N., Speyer J. L., Green M., Canetta R., Beller U., Wernz J. C., Meyers M., Widman T., Blum R. H., Piccart M. Phase II study of carboplatin in recurrent ovarian cancer: severe hematologic toxicity in previously treated patients. Cancer Chemother Pharmacol. 1989;23(5):323–328. doi: 10.1007/BF00292413. [DOI] [PubMed] [Google Scholar]
- DeGregorio M. W., Lum B. L., Holleran W. M., Wilbur B. J., Sikic B. I. Preliminary observations of intraperitoneal carboplatin pharmacokinetics during a phase I study of the Northern California Oncology Group. Cancer Chemother Pharmacol. 1986;18(3):235–238. doi: 10.1007/BF00273393. [DOI] [PubMed] [Google Scholar]
- Goel R., Cleary S. M., Horton C., Kirmani S., Abramson I., Kelly C., Howell S. B. Effect of sodium thiosulfate on the pharmacokinetics and toxicity of cisplatin. J Natl Cancer Inst. 1989 Oct 18;81(20):1552–1560. doi: 10.1093/jnci/81.20.1552. [DOI] [PubMed] [Google Scholar]
- Goel R., McClay E. F., Kirmani S., Kim S., Braly P. F., Plaxe S. C., Alcaraz J., Andrews P. A., Reichman B., Markman M. Pharmacokinetic study of intraperitoneal streptozotocin. Clin Invest Med. 1992 Oct;15(5):420–426. [PubMed] [Google Scholar]
- Gore M. E., Calvert A. H., Smith L. E. High dose carboplatin in the treatment of lung cancer and mesothelioma: a phase I dose escalation study. Eur J Cancer Clin Oncol. 1987 Sep;23(9):1391–1397. doi: 10.1016/0277-5379(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Howell S. B., Kirmani S., Lucas W. E., Zimm S., Goel R., Kim S., Horton M. C., McVey L., Morris J., Weiss R. J. A phase II trial of intraperitoneal cisplatin and etoposide for primary treatment of ovarian epithelial cancer. J Clin Oncol. 1990 Jan;8(1):137–145. doi: 10.1200/JCO.1990.8.1.137. [DOI] [PubMed] [Google Scholar]
- Markman M., Reichman B., Hakes T., Rubin S., Jones W., Lewis J. L., Jr, Barakat R., Curtin J., Almadrones L., Hoskins W. Phase 2 trial of intraperitoneal carboplatin and etoposide as salvage treatment of advanced epithelial ovarian cancer. Gynecol Oncol. 1992 Dec;47(3):353–357. doi: 10.1016/0090-8258(92)90139-a. [DOI] [PubMed] [Google Scholar]
- Meyers F. J., Welborn J., Lewis J. P., Flynn N. Infusion carboplatin treatment of relapsed and refractory acute leukemia: evidence of efficacy with minimal extramedullary toxicity at intermediate doses. J Clin Oncol. 1989 Feb;7(2):173–178. doi: 10.1200/JCO.1989.7.2.173. [DOI] [PubMed] [Google Scholar]
- O'Dwyer P. J., LaCreta F. P., Daugherty J. P., Hogan M., Rosenblum N. G., O'Dwyer J. L., Comis R. L. Phase I pharmacokinetic study of intraperitoneal etoposide. Cancer Res. 1991 Apr 15;51(8):2041–2046. [PubMed] [Google Scholar]
- Pfeifle C. E., Howell S. B., Felthouse R. D., Woliver T. B., Andrews P. A., Markman M., Murphy M. P. High-dose cisplatin with sodium thiosulfate protection. J Clin Oncol. 1985 Feb;3(2):237–244. doi: 10.1200/JCO.1985.3.2.237. [DOI] [PubMed] [Google Scholar]
- Shea T. C., Flaherty M., Elias A., Eder J. P., Antman K., Begg C., Schnipper L., Frei E., 3rd, Henner W. D. A phase I clinical and pharmacokinetic study of carboplatin and autologous bone marrow support. J Clin Oncol. 1989 May;7(5):651–661. doi: 10.1200/JCO.1989.7.5.651. [DOI] [PubMed] [Google Scholar]
- Smith I. E., Evans B. D., Gore M. E., Vincent M. D., Repetto L., Yarnold J. R., Ford H. T. Carboplatin (Paraplatin; JM8) and etoposide (VP-16) as first-line combination therapy for small-cell lung cancer. J Clin Oncol. 1987 Feb;5(2):185–189. doi: 10.1200/JCO.1987.5.2.185. [DOI] [PubMed] [Google Scholar]
- Speyer J. L., Beller U., Colombo N., Sorich J., Wernz J. C., Hochster H., Green M., Porges R., Muggia F. M., Canetta R. Intraperitoneal carboplatin: favorable results in women with minimal residual ovarian cancer after cisplatin therapy. J Clin Oncol. 1990 Aug;8(8):1335–1341. doi: 10.1200/JCO.1990.8.8.1335. [DOI] [PubMed] [Google Scholar]
- Strife R. J., Jardine I., Colvin M. Analysis of the anticancer drugs VP 16-213 and VM 26 and their metabolites by high-performance liquid chromatography. J Chromatogr. 1980 May 9;182(2):211–220. doi: 10.1016/s0378-4347(00)81625-4. [DOI] [PubMed] [Google Scholar]
- Zimm S., Cleary S. M., Lucas W. E., Weiss R. J., Markman M., Andrews P. A., Schiefer M. A., Kim S., Horton C., Howell S. B. Phase I/pharmacokinetic study of intraperitoneal cisplatin and etoposide. Cancer Res. 1987 Mar 15;47(6):1712–1716. [PubMed] [Google Scholar]
- ten Bokkel Huinink W. W., van der Burg M. E., van Oosterom A. T., Neijt J. P., George M., Guastalla J. P., Veenhof C. H., Rotmensz N., Dalesio O., Vermorken J. B. Carboplatin in combination therapy for ovarian cancer. Cancer Treat Rev. 1988 Jun;15 (Suppl B):9–15. doi: 10.1016/0305-7372(88)90030-8. [DOI] [PubMed] [Google Scholar]


