Abstract
It is well established that renal allograft recipients (RARs) have an increased incidence of viral warts and premalignant and malignant cutaneous lesions, and the risk of their development increases in proportion to duration of graft survival. It has been postulated that, in addition to the effects of prolonged immunosuppression and previous sun exposure, human papillomaviruses (HPV) may also contribute to the carcinogenic process. In this study, the prevalence of HPV DNA was examined in a range of premalignant and malignant cutaneous tumours from 50 immunosuppressed patients (47 renal allograft recipients plus three cardiac allograft recipients) and 56 immunocompetent patients using Southern hybridisation as a low-stringency screening method and type-specific polymerase chain reaction (PCR) assays for eight HPV types. The combined results for renal allograft recipients show that HPV DNA was detectable in 79% of viral warts, 42% of premalignant keratoses, 33% of intraepidermal carcinomas, 43% of invasive squamous cell carcinomas and 16% of uninvolved skin specimens (squamous cell carcinomas/renal allograft recipients significantly different at P < 0.05 from uninvolved skin specimens/renal allograft recipients). In immunocompetent patients the pattern of HPV DNA prevalence was 100% for viral warts; 25% for keratoses, 23% for intraepidermal carcinomas, 22% for squamous cell carcinomas and 8% for uninvolved skin. No single HPV type predominated in tumour specimens from either group. More tumours were found to contain HPV DNA by Southern hybridisation analysis than PCR, indicating the presence of HPV types other than HPV 1, 2, 5, 6, 8, 11, 16 and 18 in some tumours. However, 'low cancer risk' HPV types 1, 2 and 6 as well as 'high cancer risk' HPV types 5 and 16 were specifically detected by PCR in a small number of neoplasms. These data suggest that multiple HPV types may contribute to cutaneous neoplasia in RARs and that they appear to act early in the process of carcinogenesis, perhaps by functioning as tumour promoters via stimulation of cell proliferation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
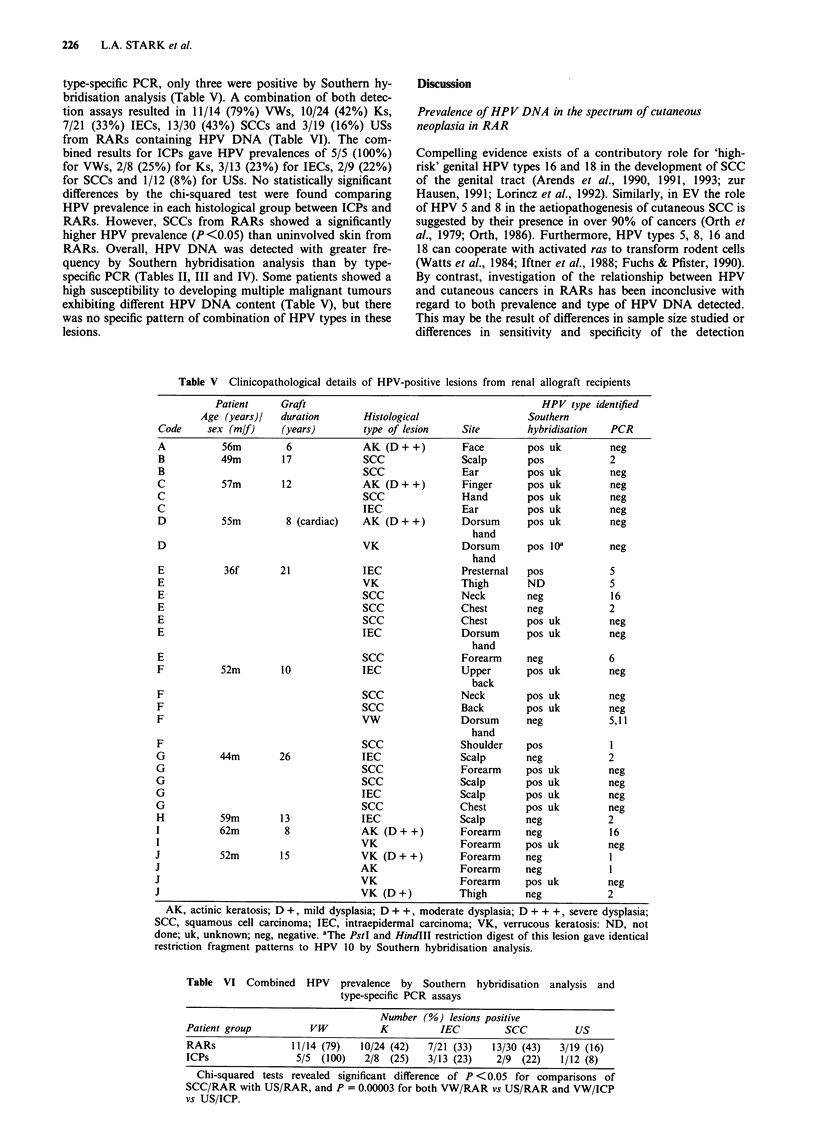
- Arends M. J., Donaldson Y. K., Duvall E., Wyllie A. H., Bird C. C. HPV in full thickness cervical biopsies: high prevalence in CIN 2 and CIN 3 detected by a sensitive PCR method. J Pathol. 1991 Dec;165(4):301–309. doi: 10.1002/path.1711650405. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Donaldson Y. K., Duvall E., Wyllie A. H., Bird C. C. Human papillomavirus type 18 associates with more advanced cervical neoplasia than human papillomavirus type 16. Hum Pathol. 1993 Apr;24(4):432–437. doi: 10.1016/0046-8177(93)90093-v. [DOI] [PubMed] [Google Scholar]
- Arends M. J., Wyllie A. H., Bird C. C. Papillomaviruses and human cancer. Hum Pathol. 1990 Jul;21(7):686–698. doi: 10.1016/0046-8177(90)90027-3. [DOI] [PubMed] [Google Scholar]
- Ashinoff R., Li J. J., Jacobson M., Friedman-Kien A. E., Geronemus R. G. Detection of human papillomavirus DNA in squamous cell carcinoma of the nail bed and finger determined by polymerase chain reaction. Arch Dermatol. 1991 Dec;127(12):1813–1818. [PubMed] [Google Scholar]
- Baadsgaard O. In vivo ultraviolet irradiation of human skin results in profound perturbation of the immune system. Relevance to ultraviolet-induced skin cancer. Arch Dermatol. 1991 Jan;127(1):99–109. [PubMed] [Google Scholar]
- Barr B. B., Benton E. C., McLaren K., Bunney M. H., Smith I. W., Blessing K., Hunter J. A. Human papilloma virus infection and skin cancer in renal allograft recipients. Lancet. 1989 Jan 21;1(8630):124–129. doi: 10.1016/s0140-6736(89)91143-4. [DOI] [PubMed] [Google Scholar]
- Birkeland S. A. Malignant tumors in renal transplant patients. The Scandia transplant material. Cancer. 1983 May 1;51(9):1571–1575. doi: 10.1002/1097-0142(19830501)51:9<1571::aid-cncr2820510903>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
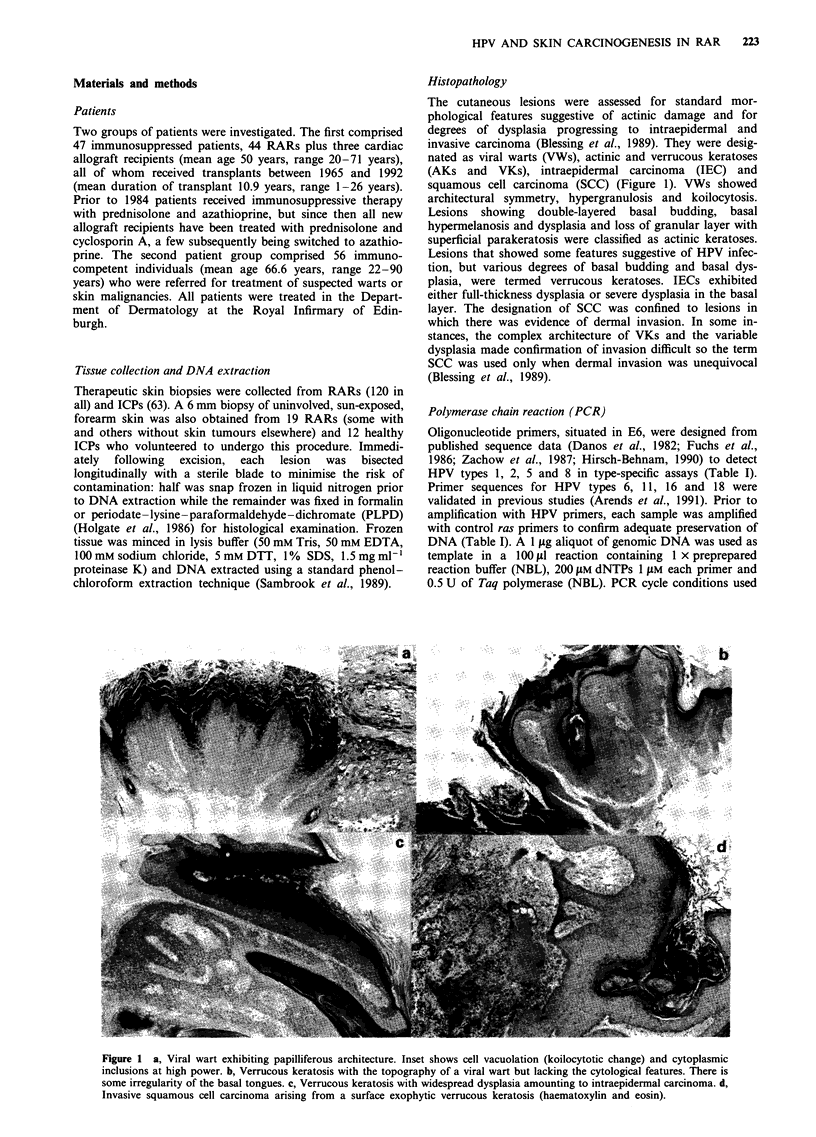
- Blessing K., McLaren K. M., Benton E. C., Barr B. B., Bunney M. H., Smith I. W., Beveridge G. W. Histopathology of skin lesions in renal allograft recipients--an assessment of viral features and dysplasia. Histopathology. 1989 Feb;14(2):129–139. doi: 10.1111/j.1365-2559.1989.tb02123.x. [DOI] [PubMed] [Google Scholar]
- Blohmé I., Larkö O. Premalignant and malignant skin lesions in renal transplant patients. Transplantation. 1984 Feb;37(2):165–167. doi: 10.1097/00007890-198402000-00010. [DOI] [PubMed] [Google Scholar]
- Boyle J., MacKie R. M., Briggs J. D., Junor B. J., Aitchison T. C. Cancer, warts, and sunshine in renal transplant patients. A case-control study. Lancet. 1984 Mar 31;1(8379):702–705. doi: 10.1016/s0140-6736(84)92221-9. [DOI] [PubMed] [Google Scholar]
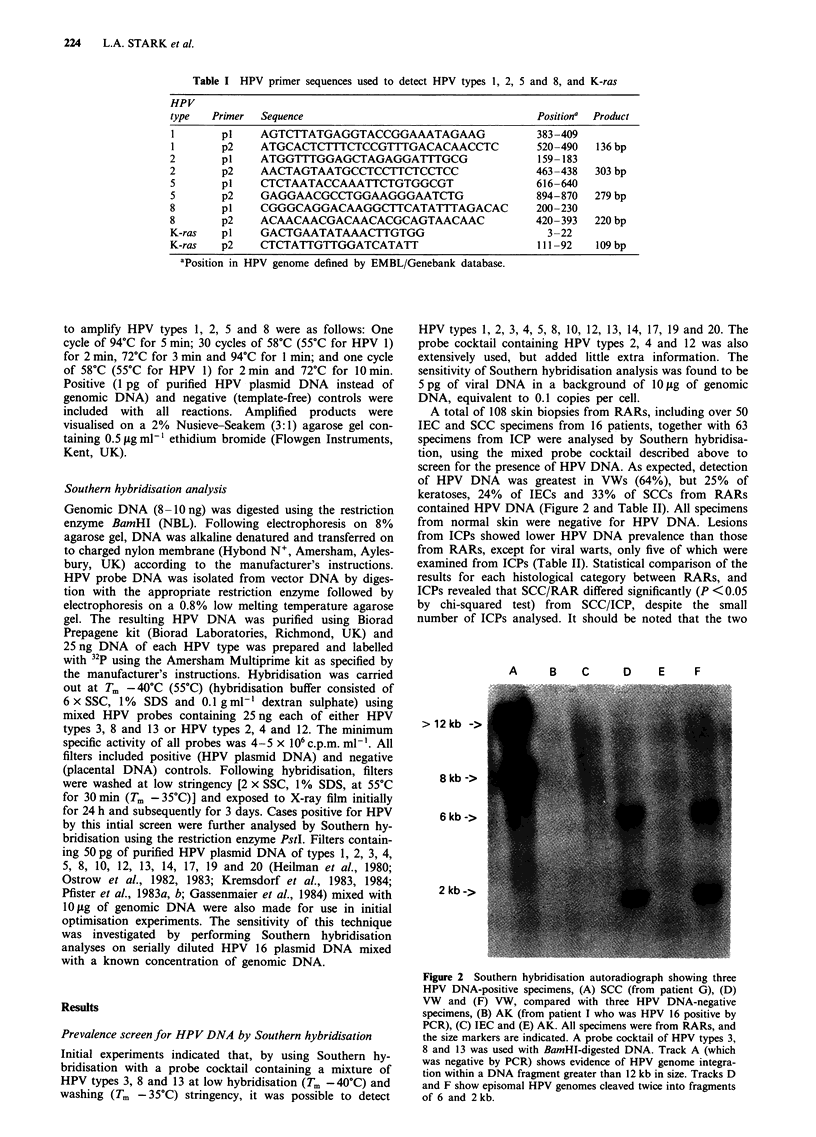
- Danos O., Katinka M., Yaniv M. Human papillomavirus 1a complete DNA sequence: a novel type of genome organization among papovaviridae. EMBO J. 1982;1(2):231–236. doi: 10.1002/j.1460-2075.1982.tb01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith D., Trowell H., Mark A., Dyall-Smith M. Cutaneous squamous cell carcinomas and papillomaviruses in renal transplant recipients: a clinical and molecular biological study. J Dermatol Sci. 1991 May;2(3):139–146. doi: 10.1016/0923-1811(91)90059-7. [DOI] [PubMed] [Google Scholar]
- Eliezri Y. D., Silverstein S. J., Nuovo G. J. Occurrence of human papillomavirus type 16 DNA in cutaneous squamous and basal cell neoplasms. J Am Acad Dermatol. 1990 Nov;23(5 Pt 1):836–842. doi: 10.1016/0190-9622(90)70299-w. [DOI] [PubMed] [Google Scholar]
- Euvrard S., Chardonnet Y., Dureau G., Hermier C., Thivolet J. Human papillomavirus type 1-associated squamous cell carcinoma in a heart transplant recipient. Arch Dermatol. 1991 Apr;127(4):559–564. [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassenmaier A., Lammel M., Pfister H. Molecular cloning and characterization of the DNAs of human papillomaviruses 19, 20, and 25 from a patient with epidermodysplasia verruciformis. J Virol. 1984 Dec;52(3):1019–1023. doi: 10.1128/jvi.52.3.1019-1023.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. A., Law M. F., Israel M. A., Howley P. M. Cloning of human papilloma virus genomic DNAs and analysis of homologous polynucleotide sequences. J Virol. 1980 Nov;36(2):395–407. doi: 10.1128/jvi.36.2.395-407.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch-Behnam A., Delius H., de Villiers E. M. A comparative sequence analysis of two human papillomavirus (HPV) types 2a and 57. Virus Res. 1990 Dec;18(1):81–97. doi: 10.1016/0168-1702(90)90091-o. [DOI] [PubMed] [Google Scholar]
- Holgate C. S., Jackson P., Pollard K., Lunny D., Bird C. C. Effect fixation on T and B lymphocyte surface membrane antigen demonstration in paraffin processed tissue. J Pathol. 1986 Aug;149(4):293–300. doi: 10.1002/path.1711490405. [DOI] [PubMed] [Google Scholar]
- Hoxtell E. O., Mandel J. S., Murray S. S., Schuman L. M., Goltz R. W. Incidence of skin carcinoma after renal transplantation. Arch Dermatol. 1977 Apr;113(4):436–438. [PubMed] [Google Scholar]
- Iftner T., Bierfelder S., Csapo Z., Pfister H. Involvement of human papillomavirus type 8 genes E6 and E7 in transformation and replication. J Virol. 1988 Oct;62(10):3655–3661. doi: 10.1128/jvi.62.10.3655-3661.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsdorf D., Favre M., Jablonska S., Obalek S., Rueda L. A., Lutzner M. A., Blanchet-Bardon C., Van Voorst Vader P. C., Orth G. Molecular cloning and characterization of the genomes of nine newly recognized human papillomavirus types associated with epidermodysplasia verruciformis. J Virol. 1984 Dec;52(3):1013–1018. doi: 10.1128/jvi.52.3.1013-1018.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremsdorf D., Jablonska S., Favre M., Orth G. Human papillomaviruses associated with epidermodysplasia verruciformis. II. Molecular cloning and biochemical characterization of human papillomavirus 3a, 8, 10, and 12 genomes. J Virol. 1983 Nov;48(2):340–351. doi: 10.1128/jvi.48.2.340-351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A. T., Reid R., Jenson A. B., Greenberg M. D., Lancaster W., Kurman R. J. Human papillomavirus infection of the cervix: relative risk associations of 15 common anogenital types. Obstet Gynecol. 1992 Mar;79(3):328–337. doi: 10.1097/00006250-199203000-00002. [DOI] [PubMed] [Google Scholar]
- Lutzner M. A., Orth G., Dutronquay V., Ducasse M. F., Kreis H., Crosnier J. Detection of human papillomavirus type 5 DNA in skin cancers of an immunosuppressed renal allograft recipient. Lancet. 1983 Aug 20;2(8347):422–424. doi: 10.1016/s0140-6736(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Lutzner M., Croissant O., Ducasse M. F., Kreis H., Crosnier J., Orth G. A potentially oncogenic human papillomavirus (HPV-5) found in two renal allograft recipients. J Invest Dermatol. 1980 Oct;75(4):353–356. doi: 10.1111/1523-1747.ep12531131. [DOI] [PubMed] [Google Scholar]
- Orth G., Jablonska S., Jarzabek-Chorzelska M., Obalek S., Rzesa G., Favre M., Croissant O. Characteristics of the lesions and risk of malignant conversion associated with the type of human papillomavirus involved in epidermodysplasia verruciformis. Cancer Res. 1979 Mar;39(3):1074–1082. [PubMed] [Google Scholar]
- Ostrow R. S., Bender M., Niimura M., Seki T., Kawashima M., Pass F., Faras A. J. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow R. S., Manias D., Mitchell A. J., Stawowy L., Faras A. J. Epidermodysplasia verruciformis. A case associated with primary lymphatic dysplasia, depressed cell-mediated immunity, and Bowen's disease containing human papillomavirus 16 DNA. Arch Dermatol. 1987 Nov;123(11):1511–1516. doi: 10.1001/archderm.123.11.1511. [DOI] [PubMed] [Google Scholar]
- Ostrow R. S., Shaver M. K., Turnquist S., Viksnins A., Bender M., Vance C., Kaye V., Faras A. J. Human papillomavirus-16 DNA in a cutaneous invasive cancer. Arch Dermatol. 1989 May;125(5):666–669. [PubMed] [Google Scholar]
- Ostrow R., Zachow K., Watts S., Bender M., Pass F., Faras A. Characterization of two HPV-3 related papillomaviruses from common warts that are distinct clinically from flat warts or epidermodysplasia verruciformis. J Invest Dermatol. 1983 May;80(5):436–440. doi: 10.1111/1523-1747.ep12555522. [DOI] [PubMed] [Google Scholar]
- Pfister H., Gassenmaier A., Nürnberger F., Stüttgen G. Human papilloma virus 5-DNA in a carcinoma of an epidermodysplasia verruciformis patient infected with various human papillomavirus types. Cancer Res. 1983 Mar;43(3):1436–1441. [PubMed] [Google Scholar]
- Pfister H., Hettich I., Runne U., Gissmann L., Chilf G. N. Characterization of human papillomavirus type 13 from focal epithelial hyperplasia Heck lesions. J Virol. 1983 Aug;47(2):363–366. doi: 10.1128/jvi.47.2.363-366.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdlinger R., Grob R. Papillomavirus infection and skin cancer in renal allograft recipients. Lancet. 1989 May 20;1(8647):1132–1133. doi: 10.1016/s0140-6736(89)92403-3. [DOI] [PubMed] [Google Scholar]
- Rüdlinger R., Grob R., Yu Y. X., Schnyder U. W. Human papillomavirus-35-positive bowenoid papulosis of the anogenital area and concurrent human papillomavirus-35-positive verruca with bowenoid dysplasia of the periungual area. Arch Dermatol. 1989 May;125(5):655–659. doi: 10.1001/archderm.1989.01670170069012. [DOI] [PubMed] [Google Scholar]
- Rüdlinger R., Smith I. W., Bunney M. H., Hunter J. A. Human papillomavirus infections in a group of renal transplant recipients. Br J Dermatol. 1986 Dec;115(6):681–692. doi: 10.1111/j.1365-2133.1986.tb06649.x. [DOI] [PubMed] [Google Scholar]
- Sheil A. G., Flavel S., Disney A. P., Mathew T. H. Cancer development in patients progressing to dialysis and renal transplantation. Transplant Proc. 1985 Apr;17(2):1685–1688. [PubMed] [Google Scholar]
- Shuttleworth D., Marks R., Griffin P. J., Salaman J. R. Dysplastic epidermal change in immunosuppressed patients with renal transplants. Q J Med. 1987 Jul;64(243):609–616. [PubMed] [Google Scholar]
- Soler C., Chardonnet Y., Euvrard S., Chignol M. C., Thivolet J. Evaluation of human papillomavirus type 5 on frozen sections of multiple lesions from transplant recipients with in situ hybridization and non-isotopic probes. Dermatology. 1992;184(4):248–253. doi: 10.1159/000247561. [DOI] [PubMed] [Google Scholar]
- Stanley M. Genital papillomaviruses, polymerase chain reaction and cervical cancer. Genitourin Med. 1990 Dec;66(6):415–417. doi: 10.1136/sti.66.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone M. S., Noonan C. A., Tschen J., Bruce S. Bowen's disease of the feet. Presence of human papillomavirus 16 DNA in tumor tissue. Arch Dermatol. 1987 Nov;123(11):1517–1520. doi: 10.1001/archderm.123.11.1517. [DOI] [PubMed] [Google Scholar]
- Van der Leest R. J., Zachow K. R., Ostrow R. S., Bender M., Pass F., Faras A. J. Human papillomavirus heterogeneity in 36 renal transplant recipients. Arch Dermatol. 1987 Mar;123(3):354–357. doi: 10.1001/archderm.123.3.354. [DOI] [PubMed] [Google Scholar]
- Watts S. L., Phelps W. C., Ostrow R. S., Zachow K. R., Faras A. J. Cellular transformation by human papillomavirus DNA in vitro. Science. 1984 Aug 10;225(4662):634–636. doi: 10.1126/science.6330900. [DOI] [PubMed] [Google Scholar]
- Yabe Y., Tanimura Y., Sakai A., Hitsumoto T., Nohara N. Molecular characteristics and physical state of human papillomavirus DNA change with progressing malignancy: studies in a patient with epidermodysplasia verruciformis. Int J Cancer. 1989 Jun 15;43(6):1022–1028. doi: 10.1002/ijc.2910430611. [DOI] [PubMed] [Google Scholar]
- Zachow K. R., Ostrow R. S., Faras A. J. Nucleotide sequence and genome organization of human papillomavirus type 5. Virology. 1987 May;158(1):251–254. doi: 10.1016/0042-6822(87)90263-7. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]