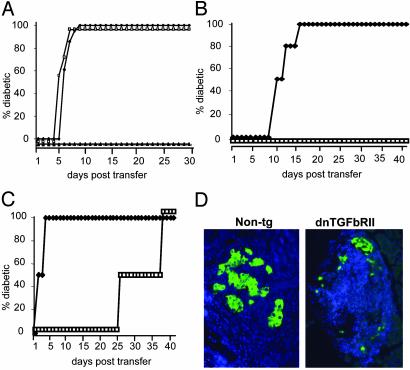
Fig. 2.
TGF-βRII-deficient CD8+ T cells cannot be controlled by islet-specific regulatory mechanisms. (A) Donor CD8+ T cells were isolated from the periLNs or the PLNs of 6-week-old dnTGFβRII tg mice. As controls, CD8+ T cells were purified from the PLNs of non-tg littermates. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age to induce regulation. On day 30, groups of recipients were injected with 3 × 104 dnTGFβRII PLN-derived (□, n = 3), periLN-derived (♦, n = 3), or non-tg PLN-derived (▴, n = 3) CD8+ T cells. The progression to diabetes in recipient mice was monitored over a 30-day observation period by measuring blood glucose levels. Mice with readings >250 mg/dl on two consecutive occasions were deemed diabetic. (B and C) Donor CD8+ T cells were isolated from the PLN of 6-week-old dnTGFβRII or non-tg mice and incubated with anti-CD8 and anti-CD44 Abs. The CD8+CD44+ and CD8+CD44– T cell populations isolated by using FACStarplus. Recipient Tet-TNF-α/CD80 mice were induced to express TNF-α from birth to 25 days of age. On day 30 the mice were injected with either naïve (B) or activated (C) 3 × 104 dnTGFβRII (♦, n = 4) or non-tg (□, n = 4) donor cells. Diabetes progression was monitored as before. The data presented are the accumulated results from the pool of two independent experiments. (D) dnTGFβRII tg CD8+ T cells rapidly destroy islet β cells. The pancreata were removed from recipient mice of non-tg CD8+CD44+ T cells or dnTGFβRII CD8+CD44+ T cells and fixed in 1% paraformaldehyde. Five- to 7-μm paraffin-embedded sections were incubated with anti-insulin Abs (green), and nuclei were stained with DAPI (blue). The data are representative of two individual mice examined from each group.