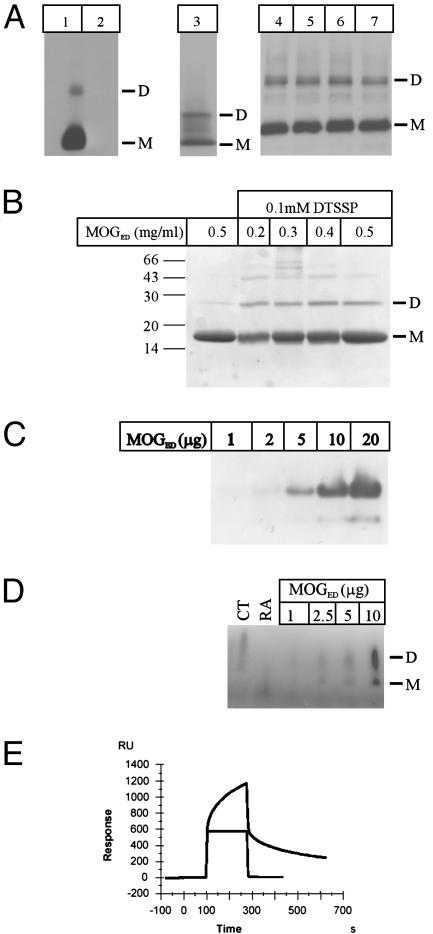
Fig. 6.
Biochemical evidence of MOG self-association. (A) Myelin purified from brain samples (lane 1, wild-type mouse; lane 2, MOG–/– mouse; lane 3, human) was separated by SDS/PAGE and immunoblotted with MOG-specific mAb 8-18C5. Purified native MOG from different human brain samples (lanes 4–7) was separated by SDS/PAGE and immunoblotted with MOG-specific mAb 8-18C5. Each of the three panels represents a separate experiment. (B) Recombinant MOGED was incubated with the cross-linker DTSSP, and the reaction was separated by SDS/PAGE. Dimer formation was observed only in the presence of the cross-linker. (C) Increasing amounts of recombinant MOGED were analyzed by nondenaturing PAGE, resulting in two species. (D) Blue native PAGE. Increasing amounts of MOGED were separated on a 4–20% native gradient PAGE gel and stained with Coomassie blue, revealing monomer dimer species. CT, chymotrypsinogen A; RA, ribonuclease A. (E) Biacore data. MOGED (10 μg/ml) was passed over biotinylated MOG35-55 (blue curve) or MOG92-106 (red curve) immobilized on a streptavidin sensor chip. Binding was analyzed by surface plasmon resonance and shows specific binding of MOGED to the 35–55 peptide. A control protein displayed no binding to either peptide (data not shown). M, monomer; D, dimer.