Abstract
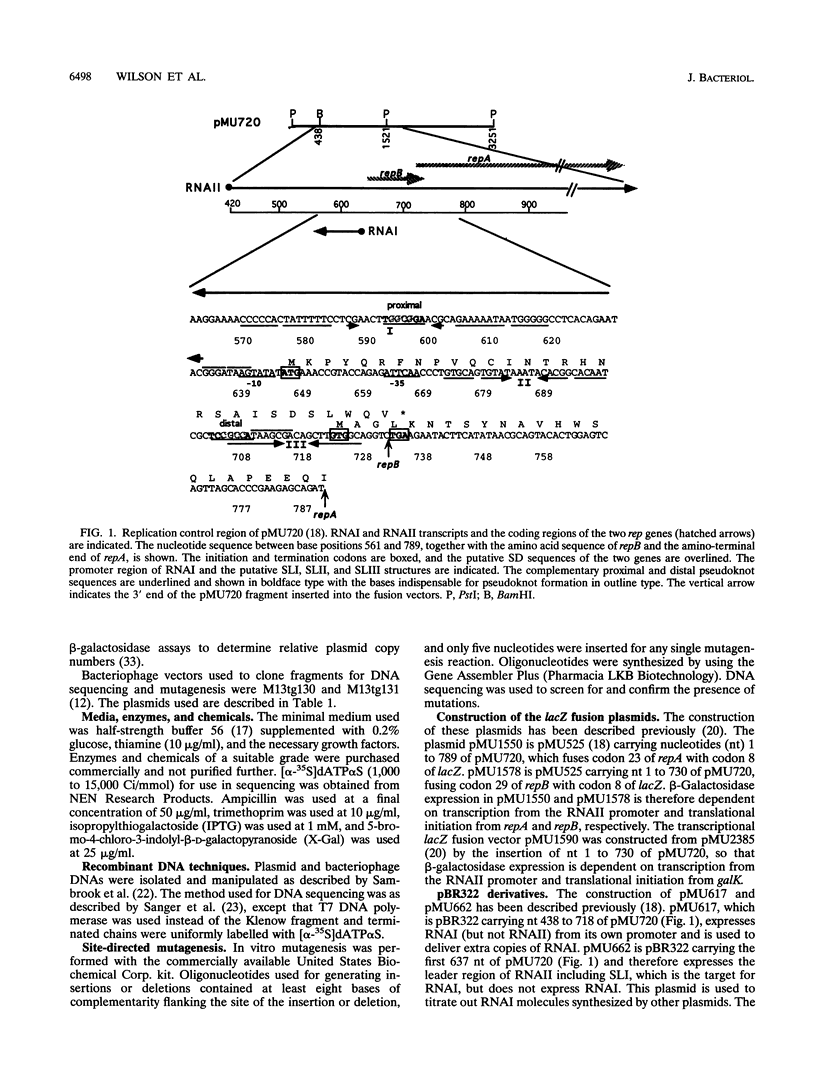
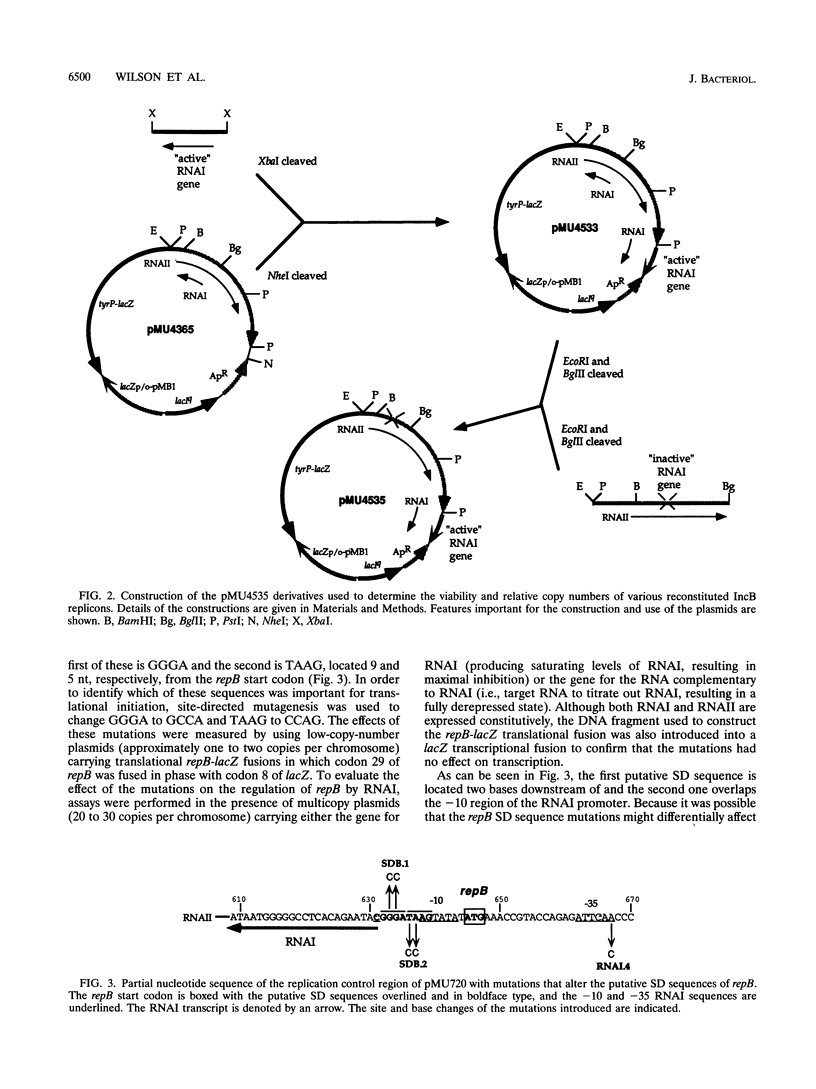
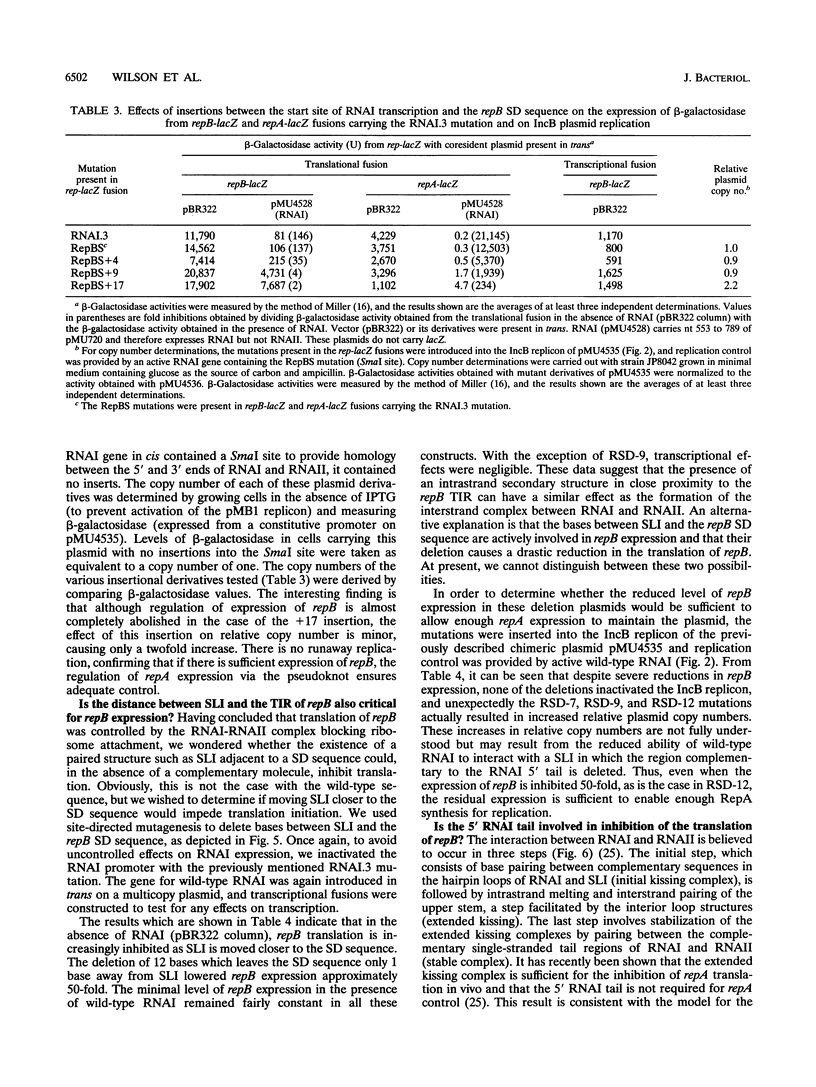
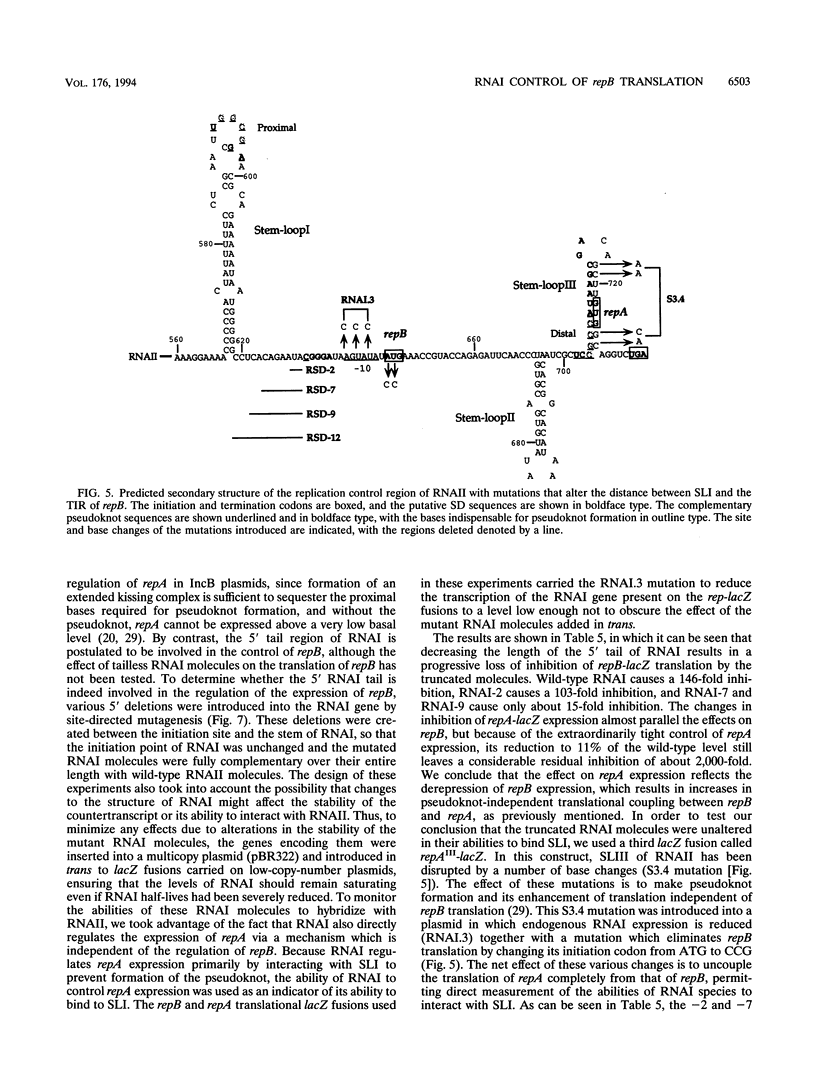
The translation of RepA, the replication initiation protein of the IncB plasmid pMU720, requires that its mRNA (RNAII) folds to form a pseudoknot immediately upstream of the repA Shine-Dalgarno sequence. The formation of this pseudoknot is dependent in turn on the translation and correct termination of a leader peptide, RepB. A small countertranscript RNA, RNAI, controls the replication of pMU720 by interacting with RNAII to negatively regulate the expression of repA both directly, by sequestering the proximal bases required for pseudoknot formation, and indirectly, by inhibiting the translation of repB. Inhibition of the translation of repB by RNAI was found to depend on the close proximity of the RNAI-RNAII complex to the translational initiation region of repB, indicating that the primary mechanism of RNAI control involves steric hindrance. Disruption of RNAI control of repB had only a small effect on the copy number of the IncB plasmid, indicating that inhibition of the expression of repA by RNAI is achieved predominantly by inhibition of pseudoknot formation rather than by inhibition of repB translation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Kato A., Moriwaki H., Hama C., Shiba K., Mizobuchi K. Positive and negative regulations of plasmid CoLIb-P9 repZ gene expression at the translational level. J Biol Chem. 1991 Feb 25;266(6):3774–3781. [PubMed] [Google Scholar]
- Asano K., Moriwaki H., Mizobuchi K. An induced mRNA secondary structure enhances repZ translation in plasmid ColIb-P9. J Biol Chem. 1991 Dec 25;266(36):24549–24556. [PubMed] [Google Scholar]
- Bird P. I., Pittard J. Demonstration of a third incompatibility function on plasmids already incompatible with group P and group I plasmids. Plasmid. 1983 Mar;9(2):191–200. doi: 10.1016/0147-619x(83)90020-3. [DOI] [PubMed] [Google Scholar]
- Blomberg P., Nordström K., Wagner E. G. Replication control of plasmid R1: RepA synthesis is regulated by CopA RNA through inhibition of leader peptide translation. EMBO J. 1992 Jul;11(7):2675–2683. doi: 10.1002/j.1460-2075.1992.tb05333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg P., Wagner E. G., Nordström K. Control of replication of plasmid R1: the duplex between the antisense RNA, CopA, and its target, CopT, is processed specifically in vivo and in vitro by RNase III. EMBO J. 1990 Jul;9(7):2331–2340. doi: 10.1002/j.1460-2075.1990.tb07405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case C. C., Roels S. M., Jensen P. D., Lee J., Kleckner N., Simons R. W. The unusual stability of the IS10 anti-sense RNA is critical for its function and is determined by the structure of its stem-domain. EMBO J. 1989 Dec 20;8(13):4297–4305. doi: 10.1002/j.1460-2075.1989.tb08616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X. N., Womble D. D., Rownd R. H. Transcriptional pausing in a region important for plasmid NR1 replication control. J Bacteriol. 1987 Dec;169(12):5353–5363. doi: 10.1128/jb.169.12.5353-5363.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Womble D. D., Luckow V. A., Rownd R. H. Regulation of transcription of the repA1 gene in the replication control region of IncFII plasmid NR1 by gene dosage of the repA2 transcription repressor protein. J Bacteriol. 1985 Feb;161(2):544–551. doi: 10.1128/jb.161.2.544-551.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil D., Bouché J. P. ColE1-type vectors with fully repressible replication. Gene. 1991 Aug 30;105(1):17–22. doi: 10.1016/0378-1119(91)90508-9. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Improved predictions of secondary structures for RNA. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7706–7710. doi: 10.1073/pnas.86.20.7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Krinke L., Wulff D. L. OOP RNA, produced from multicopy plasmids, inhibits lambda cII gene expression through an RNase III-dependent mechanism. Genes Dev. 1987 Nov;1(9):1005–1013. doi: 10.1101/gad.1.9.1005. [DOI] [PubMed] [Google Scholar]
- Light J., Riise E., Molin S. Transcription and its regulation in the basic replicon region of plasmid R1. Mol Gen Genet. 1985;198(3):503–508. doi: 10.1007/BF00332947. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Praszkier J., Bird P., Nikoletti S., Pittard J. Role of countertranscript RNA in the copy number control system of an IncB miniplasmid. J Bacteriol. 1989 Sep;171(9):5056–5064. doi: 10.1128/jb.171.9.5056-5064.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praszkier J., Wei T., Siemering K., Pittard J. Comparative analysis of the replication regions of IncB, IncK, and IncZ plasmids. J Bacteriol. 1991 Apr;173(7):2393–2397. doi: 10.1128/jb.173.7.2393-2397.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praszkier J., Wilson I. W., Pittard A. J. Mutations affecting translational coupling between the rep genes of an IncB miniplasmid. J Bacteriol. 1992 Apr;174(7):2376–2383. doi: 10.1128/jb.174.7.2376-2383.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringquist S., Shinedling S., Barrick D., Green L., Binkley J., Stormo G. D., Gold L. Translation initiation in Escherichia coli: sequences within the ribosome-binding site. Mol Microbiol. 1992 May;6(9):1219–1229. doi: 10.1111/j.1365-2958.1992.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering K. R., Praszkier J., Pittard A. J. Interaction between the antisense and target RNAs involved in the regulation of IncB plasmid replication. J Bacteriol. 1993 May;175(10):2895–2906. doi: 10.1128/jb.175.10.2895-2906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering K. R., Praszkier J., Pittard A. J. Mechanism of binding of the antisense and target RNAs involved in the regulation of IncB plasmid replication. J Bacteriol. 1994 May;176(9):2677–2688. doi: 10.1128/jb.176.9.2677-2688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Vandeyar M. A., Weiner M. P., Hutton C. J., Batt C. A. A simple and rapid method for the selection of oligodeoxynucleotide-directed mutants. Gene. 1988 May 15;65(1):129–133. doi: 10.1016/0378-1119(88)90425-8. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Blomberg P., Nordström K. Replication control in plasmid R1: duplex formation between the antisense RNA, CopA, and its target, CopT, is not required for inhibition of RepA synthesis. EMBO J. 1992 Mar;11(3):1195–1203. doi: 10.1002/j.1460-2075.1992.tb05160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson I. W., Praszkier J., Pittard A. J. Mutations affecting pseudoknot control of the replication of B group plasmids. J Bacteriol. 1993 Oct;175(20):6476–6483. doi: 10.1128/jb.175.20.6476-6483.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womble D. D., Sampathkumar P., Easton A. M., Luckow V. A., Rownd R. H. Transcription of the replication control region of the IncFII R-plasmid NR1 in vitro and in vivo. J Mol Biol. 1985 Feb 5;181(3):395–410. doi: 10.1016/0022-2836(85)90228-1. [DOI] [PubMed] [Google Scholar]
- Wu R., Wang X., Womble D. D., Rownd R. H. Expression of the repA1 gene of IncFII plasmid NR1 is translationally coupled to expression of an overlapping leader peptide. J Bacteriol. 1992 Dec;174(23):7620–7628. doi: 10.1128/jb.174.23.7620-7628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Wang X., Womble D. D., Rownd R. H. Suppression of replication-deficient mutants of IncFII plasmid NR1 can occur by two different mechanisms that increase expression of the repA1 gene. J Bacteriol. 1993 May;175(10):3161–3173. doi: 10.1128/jb.175.10.3161-3173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ganesan S., Sarsero J., Pittard A. J. A genetic analysis of various functions of the TyrR protein of Escherichia coli. J Bacteriol. 1993 Mar;175(6):1767–1776. doi: 10.1128/jb.175.6.1767-1776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Pittard J. Molecular analysis of the regulatory region of the Escherichia coli K-12 tyrB gene. J Bacteriol. 1987 Oct;169(10):4710–4715. doi: 10.1128/jb.169.10.4710-4715.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuker M. On finding all suboptimal foldings of an RNA molecule. Science. 1989 Apr 7;244(4900):48–52. doi: 10.1126/science.2468181. [DOI] [PubMed] [Google Scholar]


