Abstract
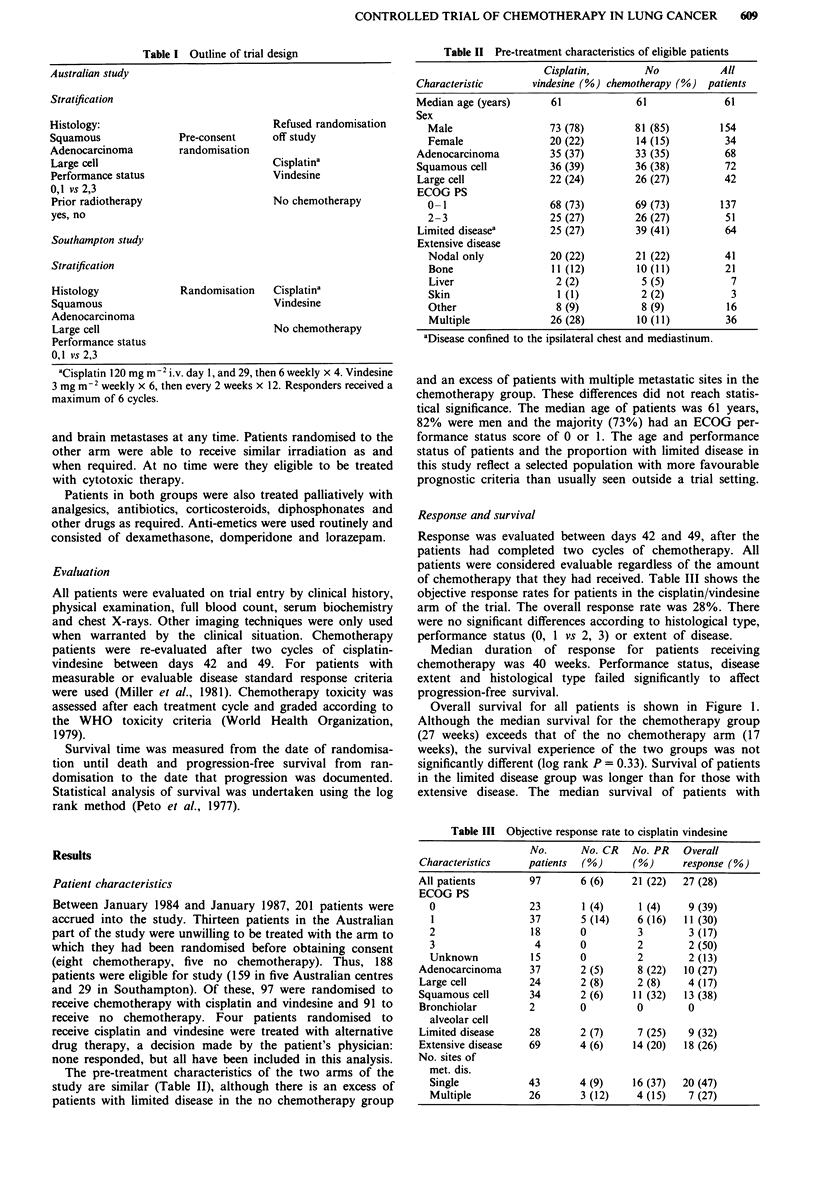
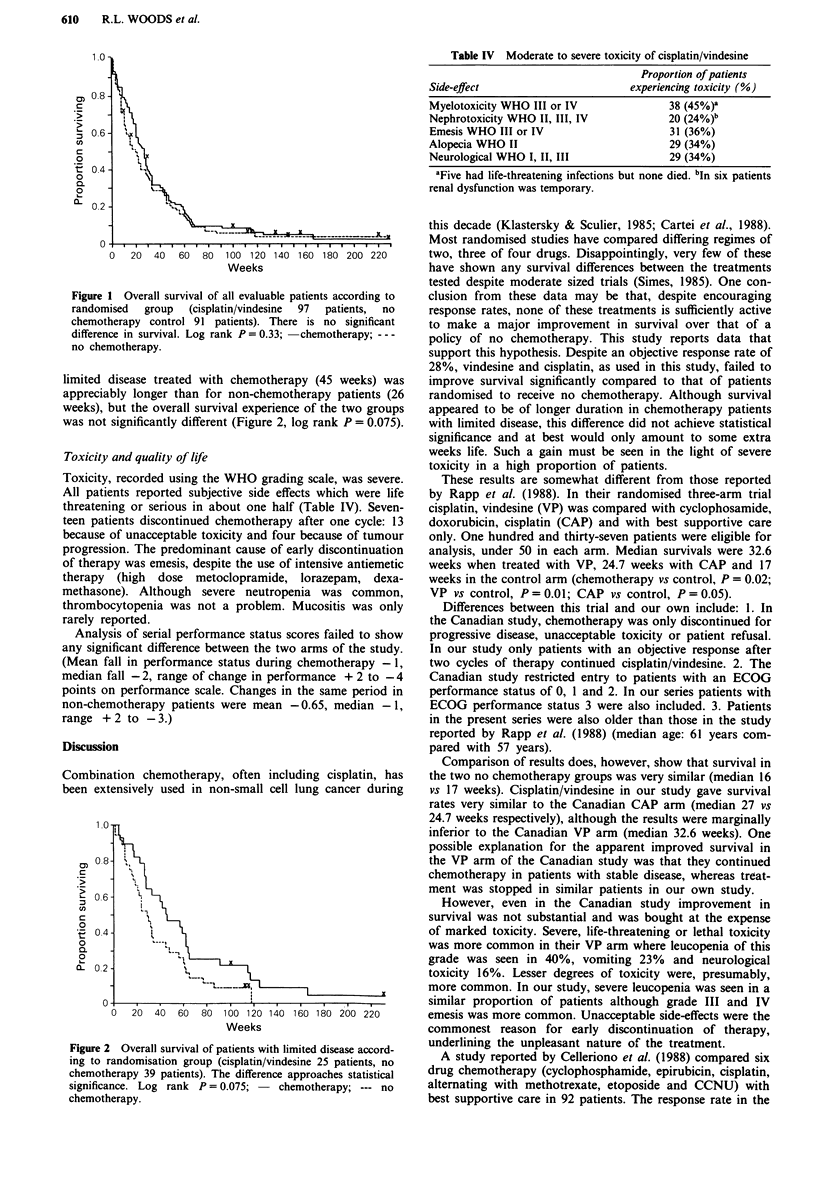
The value of chemotherapy in advanced non-small cell lung cancer (NSCLC) remains contentious. Because of this two separate but very similar trials were set up in Australia and Southampton (UK). Two hundred and one patients with stage IIIb or IV NSCLC were randomly assigned to cisplatin 120 mg m-2 on days 1 and 29 and vindesine 3 mg m-2 weekly x 6 or to no chemotherapy. Both groups were eligible to receive radiotherapy or other palliative treatment as required. Of 188 evaluable patients, 97 received chemotherapy and 91 were in the control arm. Response was assessed between days 42 and 49. Responders continued chemotherapy at the same doses though cisplatin being given 6 weekly x 4 and the vindesine 2 weekly x 12. The overall response rate to chemotherapy was 28%; there were no significant differences according to major prognostic criteria. Although the overall survival of the chemotherapy group (median 27 weeks) was longer than that of the no chemotherapy group (median 17 weeks) this was not statistically significant (log rank P = 0.33). For patients without dissemination (IIIb), median survival was 45 weeks in the chemotherapy arm and 26 weeks in the non-chemotherapy (log rank P = 0.075). Toxicity was universal and frequently severe: of 17 patients discontinuing chemotherapy after one cycle, 13 did so because of unacceptable toxicity. This chemotherapy cannot be recommended as routine treatment. Further phase III studies of chemotherapy in advanced NSCLC should continue to use a no chemotherapy control and should also attempt to measure quality of life, an issue not addressed effectively in this or other recent trials.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cellerino R., Tummarello D., Porfiri E., Guidi F., Isidori P., Raspugli M., Biscottini B., Fatati G. Non small cell lung cancer (NSCLC). A prospective randomized trial with alternating chemotherapy CEP/MEC' versus no treatment. Eur J Cancer Clin Oncol. 1988 Dec;24(12):1839–1843. doi: 10.1016/0277-5379(88)90095-8. [DOI] [PubMed] [Google Scholar]
- Cormier Y., Bergeron D., La Forge J., Lavandier M., Fournier M., Chenard J., Desmeules M. Benefits of polychemotherapy in advanced non-small-cell bronchogenic carcinoma. Cancer. 1982 Sep 1;50(5):845–849. doi: 10.1002/1097-0142(19820901)50:5<845::aid-cncr2820500507>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Dhingra H. M., Valdivieso M., Carr D. T., Chiuten D. F., Farha P., Murphy W. K., Spitzer G., Umsawasdi T. Randomized trial of three combinations of cisplatin with vindesine and/or VP-16-213 in the treatment of advanced non-small-cell lung cancer. J Clin Oncol. 1985 Feb;3(2):176–183. doi: 10.1200/JCO.1985.3.2.176. [DOI] [PubMed] [Google Scholar]
- Durrant K. R., Berry R. J., Ellis F., Ridehalgh F. R., Black J. M., Hamilton W. S. Comparison of treatment policies in inoperable bronchial carcinoma. Lancet. 1971 Apr 10;1(7702):715–719. doi: 10.1016/s0140-6736(71)91986-6. [DOI] [PubMed] [Google Scholar]
- Gralla R. J., Casper E. S., Kelsen D. P., Braun D. W., Jr, Dukeman M. E., Martini N., Young C. W., Golbey R. B. Cisplatin and vindesine combination chemotherapy for advanced carcinoma of the lung: A randomized trial investigating two dosage schedules. Ann Intern Med. 1981 Oct;95(4):414–420. doi: 10.7326/0003-4819-95-4-414. [DOI] [PubMed] [Google Scholar]
- Hoffman P. C., Bitran J. D., Golomb H. M. Chemotherapy of metastatic non-small cell bronchogenic carcinoma. Semin Oncol. 1983 Mar;10(1):111–122. [PubMed] [Google Scholar]
- Klastersky J., Sculier J. P. Chemotherapy of non-small-cell lung cancer. Semin Oncol. 1985 Dec;12(4 Suppl 6):38–48. [PubMed] [Google Scholar]
- Laing A. H., Berry R. J., Newman C. R., Peto J. Treatment of inoperable carcinoma of bronchus. Lancet. 1975 Dec 13;2(7946):1161–1164. doi: 10.1016/s0140-6736(75)92654-9. [DOI] [PubMed] [Google Scholar]
- Mackillop W. J., Ward G. K., O'Sullivan B. The use of expert surrogates to evaluate clinical trials in non-small cell lung cancer. Br J Cancer. 1986 Oct;54(4):661–667. doi: 10.1038/bjc.1986.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rapp E., Pater J. L., Willan A., Cormier Y., Murray N., Evans W. K., Hodson D. I., Clark D. A., Feld R., Arnold A. M. Chemotherapy can prolong survival in patients with advanced non-small-cell lung cancer--report of a Canadian multicenter randomized trial. J Clin Oncol. 1988 Apr;6(4):633–641. doi: 10.1200/JCO.1988.6.4.633. [DOI] [PubMed] [Google Scholar]
- Simes R. J. Risk-benefit relationships in cancer clinical trials: the ECOG experience in non-small-cell lung cancer. J Clin Oncol. 1985 Apr;3(4):462–472. doi: 10.1200/JCO.1985.3.4.462. [DOI] [PubMed] [Google Scholar]


