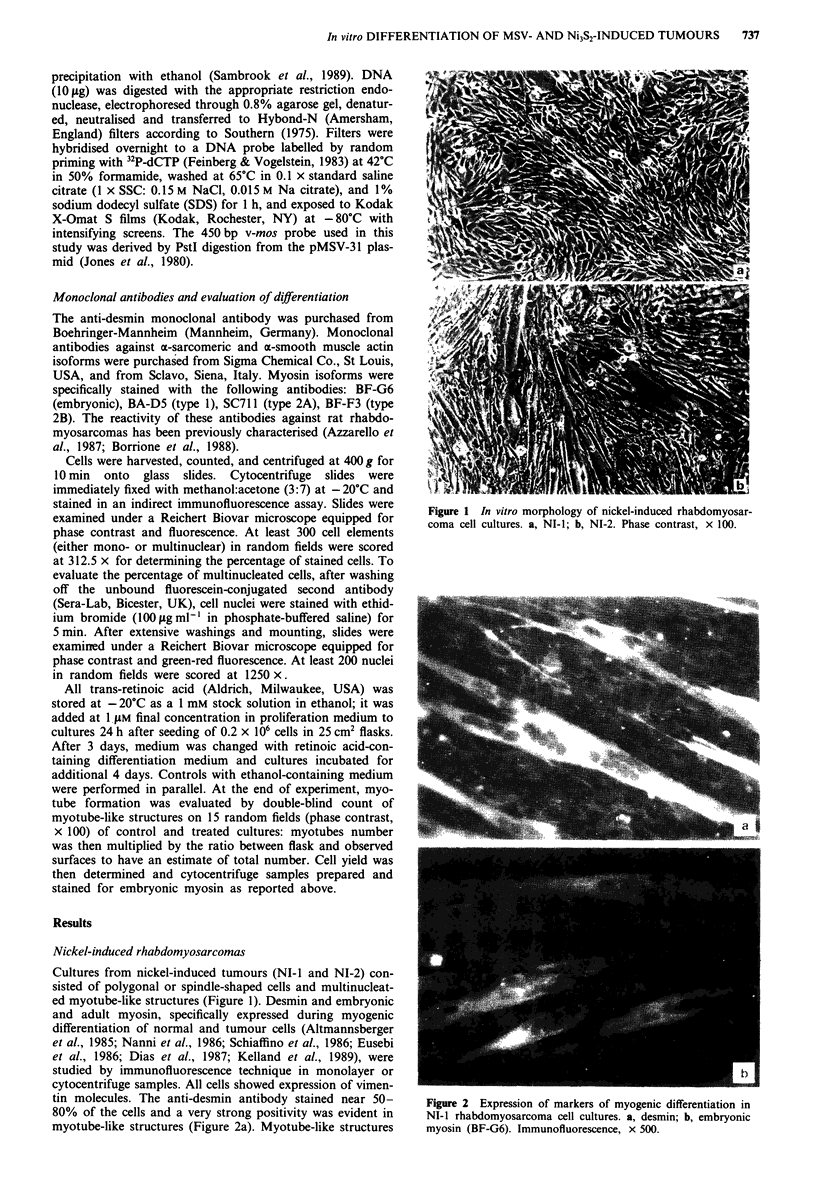
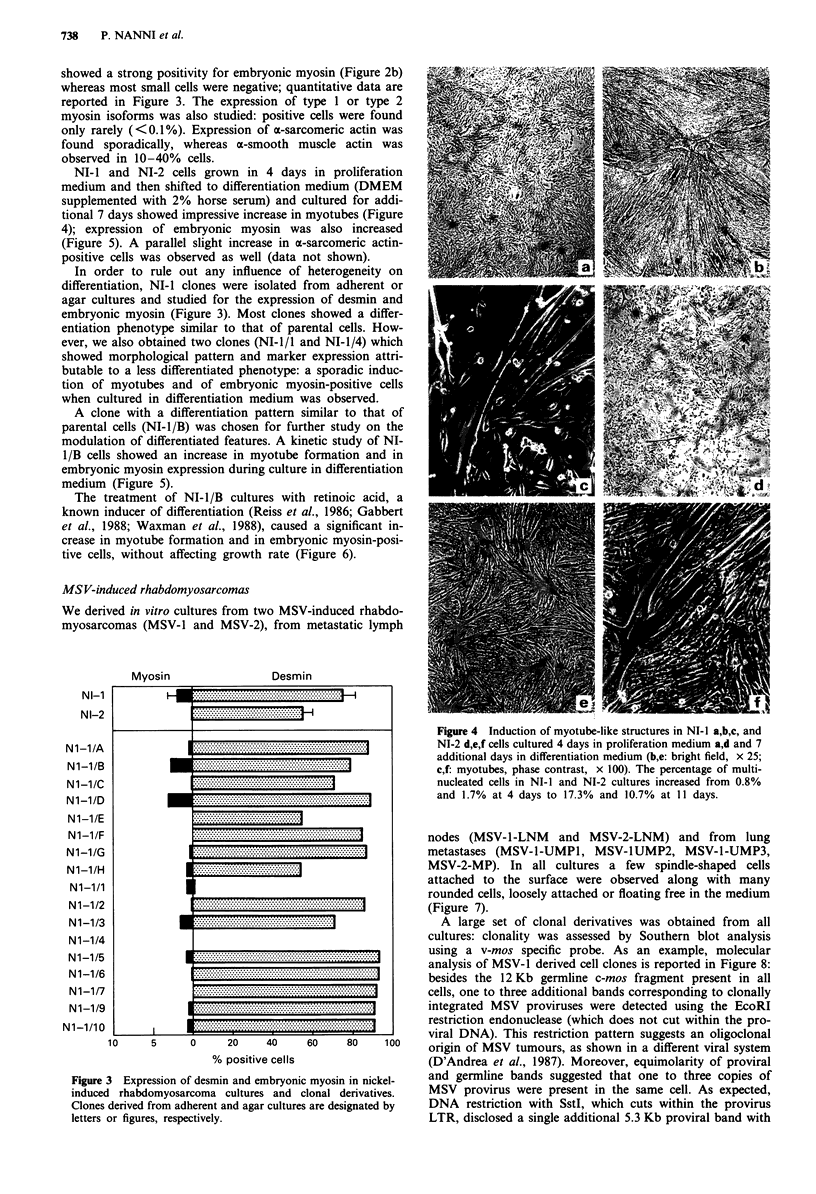
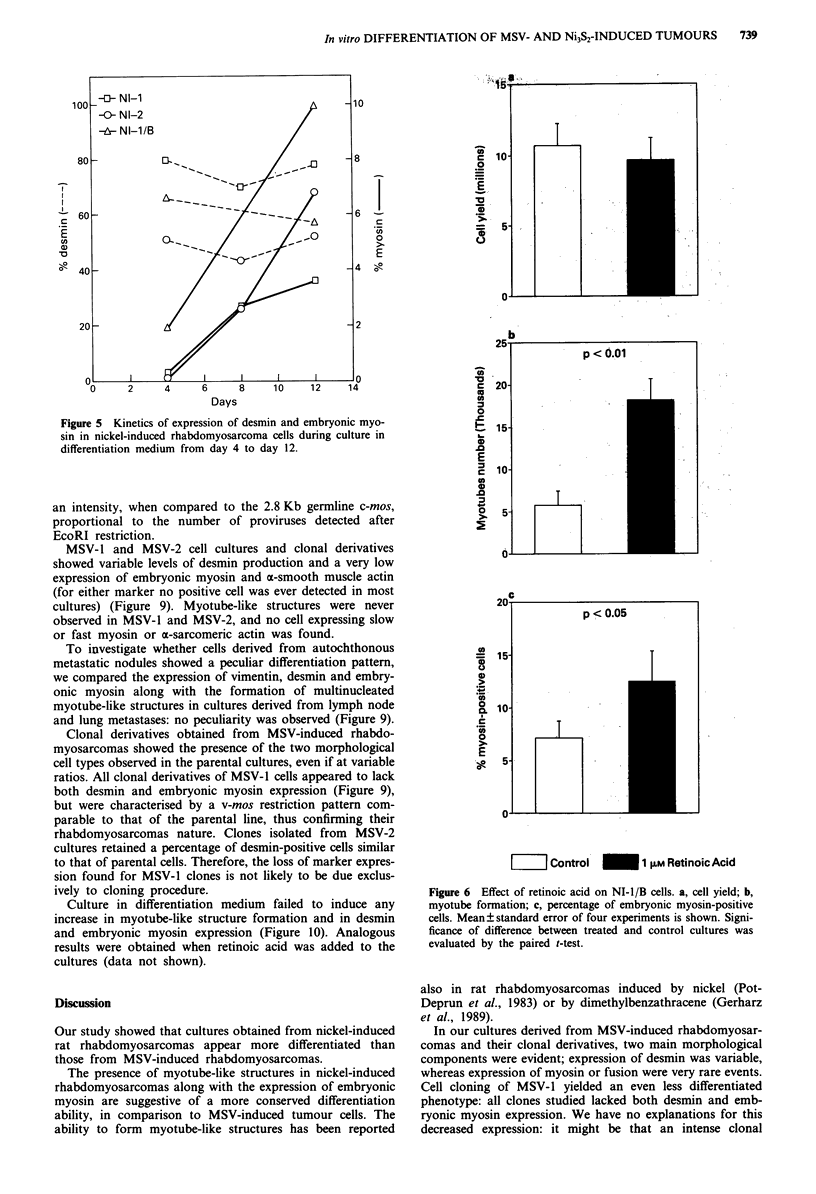
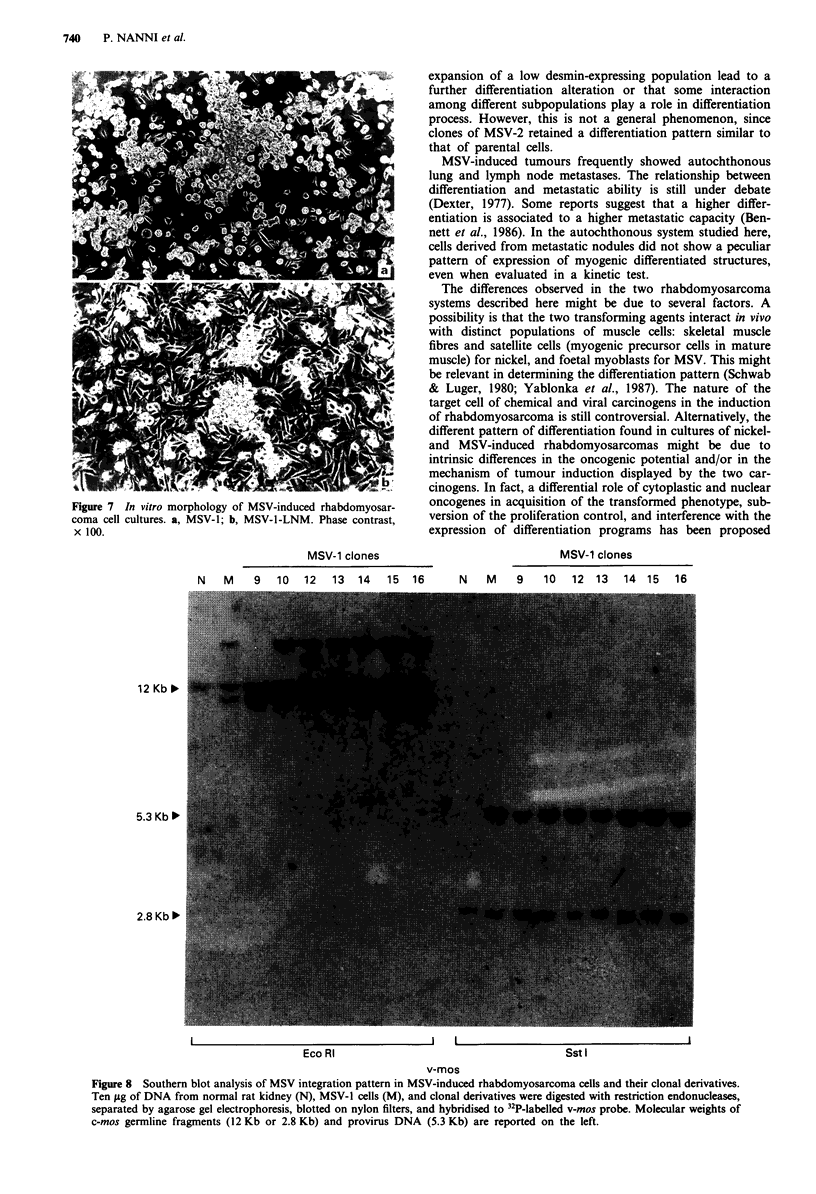
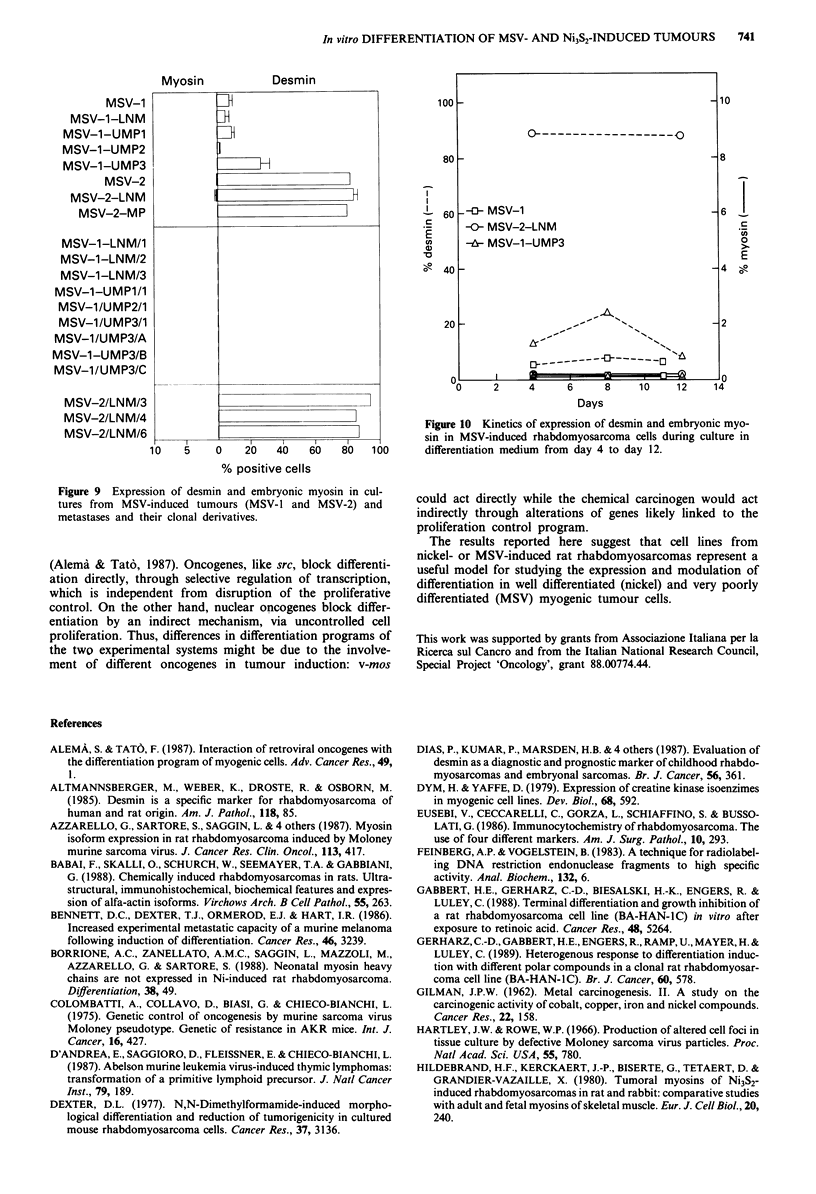
Abstract
In vitro cultures and clonal derivatives have been established from rat rhabdomyosarcomas induced by Moloney-Murine Sarcoma Virus (MSV) or by nickel sulfide; differentiation ability has been studied as expression of desmin, embryonic and adult myosin isoforms, alpha-actin isoforms and cellular fusion. The two rhabdomyosarcoma models showed different levels of myogenic differentiation. Multinucleated myotube-like structures were frequently observed in cultures derived from nickel-induced tumours. Desmin was present in 50-80% of cells and embryonic myosin in up to 10%. In MSV-tumour-derived cultures and in their metastases or clonal derivatives two cell types are present in different ratios: spindle-shaped cells, adherent to plastic surfaces, and rounded cells, loosely attached or floating free in the medium. These cultures showed features of myogenic differentiation (10-80% desmin-positive cells), but embryonic myosin expression and production of multinucleated myotube-like structures were very rare events. Cultures from autochthonous lymph node and lung metastatic cells showed similar patterns of differentiation. Retinoic acid increased differentiated features (myotube formation and embryonic myosin expression) only in nickel-induced rhabdomyosarcoma cells. The two models described here mimic the heterogeneity in differentiation pattern found among human rhabdomyosarcomas. Myogenic differentiation ability was retained at a good level by nickel-induced tumours, whereas it was strongly impaired in MSV-induced tumours.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemá S., Tató F. Interaction of retroviral oncogenes with the differentiation program of myogenic cells. Adv Cancer Res. 1987;49:1–28. doi: 10.1016/s0065-230x(08)60792-7. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Weber K., Droste R., Osborn M. Desmin is a specific marker for rhabdomyosarcomas of human and rat origin. Am J Pathol. 1985 Jan;118(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Azzarello G., Sartore S., Saggin L., Gorza L., D'Andrea E., Chieco-Bianchi L., Schiaffino S. Myosin isoform expression in rat rhabdomyosarcoma induced by Moloney murine sarcoma virus. J Cancer Res Clin Oncol. 1987;113(5):417–429. doi: 10.1007/BF00390035. [DOI] [PubMed] [Google Scholar]
- Babai F., Skalli O., Schurch W., Seemayer T. A., Gabbiani G. Chemically induced rhabdomyosarcomas in rats. Ultrastructural, immunohistochemical, biochemical features and expression of alpha-actin isoforms. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55(5):263–277. [PubMed] [Google Scholar]
- Bennett D. C., Dexter T. J., Ormerod E. J., Hart I. R. Increased experimental metastatic capacity of a murine melanoma following induction of differentiation. Cancer Res. 1986 Jul;46(7):3239–3244. [PubMed] [Google Scholar]
- Borrione A. C., Zanellato A. M., Saggin L., Mazzoli M., Azzarello G., Sartore S. Neonatal myosin heavy chains are not expressed in Ni-induced rat rhabdomyosarcoma. Differentiation. 1988 Jun;38(1):49–59. doi: 10.1111/j.1432-0436.1988.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Colombatti A., Collavo D., Biasi G., Chieco-Bianchi L. Genetic control of oncogenesis by murine sarcoma virus Moloney pseudotype. I. Genetics of resistance in AKR mice. Int J Cancer. 1975 Sep 15;16(3):427–434. doi: 10.1002/ijc.2910160309. [DOI] [PubMed] [Google Scholar]
- D'Andrea E., Saggioro D., Fleissner E., Chieco-Bianchi L. Abelson murine leukemia virus-induced thymic lymphomas: transformation of a primitive lymphoid precursor. J Natl Cancer Inst. 1987 Jul;79(1):189–195. [PubMed] [Google Scholar]
- Dexter D. L. N,N-Dimethylformamide-induced morphological differentiation and reduction of tumorigenicity in cultured mouse rhabdomyosarcoma cells. Cancer Res. 1977 Sep;37(9):3136–3140. [PubMed] [Google Scholar]
- Dias P., Kumar P., Marsden H. B., Morris-Jones P. H., Birch J., Swindell R., Kumar S. Evaluation of desmin as a diagnostic and prognostic marker of childhood rhabdomyosarcomas and embryonal sarcomas. Br J Cancer. 1987 Sep;56(3):361–365. doi: 10.1038/bjc.1987.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym H., Yaffe D. Expression of creatine kinase isoenzymes in myogenic cell lines. Dev Biol. 1979 Feb;68(2):592–599. doi: 10.1016/0012-1606(79)90229-x. [DOI] [PubMed] [Google Scholar]
- Eusebi V., Ceccarelli C., Gorza L., Schiaffino S., Bussolati G. Immunocytochemistry of rhabdomyosarcoma. The use of four different markers. Am J Surg Pathol. 1986 Apr;10(4):293–299. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- GILMAN J. P. Metal carcinogenesis. II. A study on the carcinogenic activity of cobalt, copper, iron, and nickel compounds. Cancer Res. 1962 Feb;22:158–162. [PubMed] [Google Scholar]
- Gabbert H. E., Gerharz C. D., Biesalski H. K., Engers R., Luley C. Terminal differentiation and growth inhibition of a rat rhabdomyosarcoma cell line (BA-HAN-1C) in vitro after exposure to retinoic acid. Cancer Res. 1988 Sep 15;48(18):5264–5269. [PubMed] [Google Scholar]
- Gerharz C. D., Gabbert H. E., Engers R., Ramp U., Mayer H., Luley C. Heterogeneous response to differentiation induction with different polar compounds in a clonal rat rhabdomyosarcoma cell line (BA-HAN-1C). Br J Cancer. 1989 Oct;60(4):578–584. doi: 10.1038/bjc.1989.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand H. F., Kerckaert J. P., Biserte G., Tetaert D., Grandier-Vazeille X. Tumoral myosins of Ni3S2-induced rhabdomyosarcomas in rat and rabbit: comparative studies with adult and fetal myosins of skeletal muscle. Eur J Cell Biol. 1980 Feb;20(3):240–248. [PubMed] [Google Scholar]
- Jones M., Bosselman R. A., van der Hoorn F. A., Berns A., Fan H., Verma I. M. Identification and molecular cloning of Moloney mouse sarcoma virus-specific sequences from uninfected mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2651–2655. doi: 10.1073/pnas.77.5.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland L. R., Bingle L., Edwards S., Steel G. G. High intrinsic radiosensitivity of a newly established and characterised human embryonal rhabdomyosarcoma cell line. Br J Cancer. 1989 Feb;59(2):160–164. doi: 10.1038/bjc.1989.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni P., Schiaffino S., De Giovanni C., Nicoletti G., Prodi G., Del Re B., Eusebi V., Ceccarelli C., Saggin L., Lollini P. L. RMZ: a new cell line from a human alveolar rhabdomyosarcoma. In vitro expression of embryonic myosin. Br J Cancer. 1986 Dec;54(6):1009–1014. doi: 10.1038/bjc.1986.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk K., Shachat D. A., Moloney J. B. Pathogenesis of a rhabdomyosarcoma (undifferentiated type) in rats induced by a murine sarcoma virus (Moloney). Cancer Res. 1968 Jun;28(6):1197–1206. [PubMed] [Google Scholar]
- Pot-Deprun J., Poupon M. F., Sweeney F. L., Chouroulinkov I. Growth, metastasis, immunogenicity, and chromosomal content of a nickel-induced rhabdomyosarcoma and subsequent cloned cell lines in rats. J Natl Cancer Inst. 1983 Dec;71(6):1241–1245. [PubMed] [Google Scholar]
- Reiss M., Gamba-Vitalo C., Sartorelli A. C. Induction of tumor cell differentiation as a therapeutic approach: preclinical models for hematopoietic and solid neoplasms. Cancer Treat Rep. 1986 Jan;70(1):201–218. [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res. 1986 Mar;163(1):211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Schwab I. A., Luger O. Reinitiation of DNA synthesis in postmitotic nuclei of myotubes by virus-mediated fusion with embryonic fibroblasts. Differentiation. 1980 Apr;16(2):93–99. doi: 10.1111/j.1432-0436.1980.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Sen P., Costa M. Induction of chromosomal damage in Chinese hamster ovary cells by soluble and particulate nickel compounds: preferential fragmentation of the heterochromatic long arm of the X-chromosome by carcinogenic crystalline NiS particles. Cancer Res. 1985 May;45(5):2320–2325. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tomatis L., Aitio A., Wilbourn J., Shuker L. Human carcinogens so far identified. Jpn J Cancer Res. 1989 Sep;80(9):795–807. doi: 10.1111/j.1349-7006.1989.tb01717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z., Quinn L. S., Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol. 1987 Jan;119(1):252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]