Figure 5.
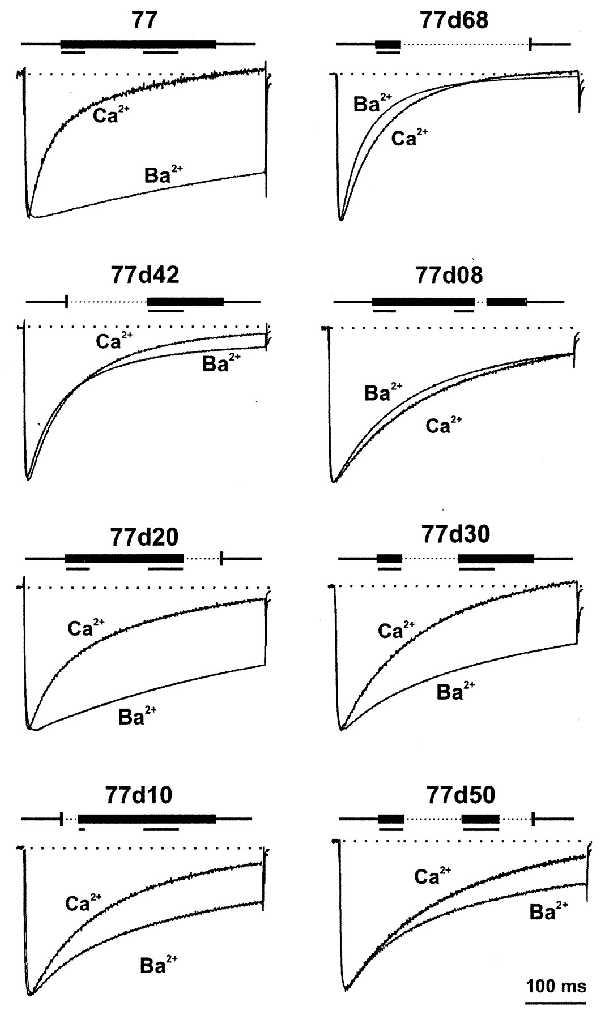
IBa and ICa traces of selected α1C constructs. A 400-ms pulse was applied from Vh = −90 mV to potentials that evoked approximately maximal IBa at +20 mV for 77, 77d20, 77d30, 77d10, 77d50, and at +30 mV for 77d68, 77d42, and 77d08. After switching from a 40 mM Ba2+- to 40 mM Ca2+-containing solution, the same TPs were applied. ICa traces were scaled to the amplitude of the corresponding IBa trace to compare time courses of IBa and ICa. Deletions of C-terminal amino acid residues between positions 1572 and 1651 for each construct are shown above each pair of current traces. Stretches of amino acids in the sequences 1572–1584 and 1614–1631 that were found to be important for Ca2+-dependent inactivation in each construct (Fig. 1) are underlined.
