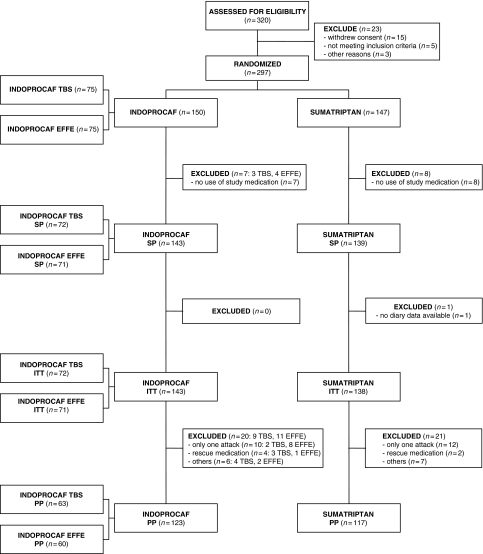
Figure 1.
Profile of subject disposition during the course of the study and inclusion in the analysis data sets: safety population (SP), intention-to-treat sample (ITT), per-protocol sample (PP)*. *Four patients treated with Indoprocaf, three with TBS (adverse event = 2 and withdrew consent = 1) and one with EFFE (adverse event), and three patients treated with sumatriptan (adverse event = 1, withdrew consent = 1 and lost to follow-up = 1) discontinued at any time during the study