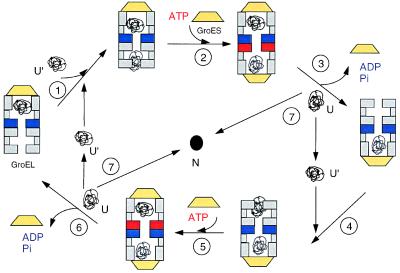
Figure 5.
Model for the reaction cycle of GroE. A cis-bullet particle with GroES, ADP, and nonnative protein (shown in black) attached to one GroEL ring binds a second folding intermediate (shown in gray) to the high-affinity binding site of the trans-ring (step 1). In a next step, ATP and a second GroES associate to form a football-shaped particle (step 2) encapsulating two folding intermediates. Hydrolysis of ATP in lower GroEL ring releases GroES, ADP, and protein from the upper ring (step 3), thus completing the flip-flop and restoring a new high-affinity acceptor state; however, it is in a rotated state. The released nonnative protein can either fold to the native state (N; step 7) or rebind to the chaperonin (step 1). To achieve catalysis, the conformations of the intermediate dissociated from GroEL and bound to GroEL have to differ with U representing an early folding intermediate and U′ a kinetically trapped species. The protein released from GroEL (U) will either fold to the native state (N) in a fast reaction or convert again to (U′) and undergo another unfolding cycle. A set of reactions (steps 4–6) equivalent to steps 1–3 completes the cycle and leads back to the original orientation. The GroEL domain complexed with ATP is shown in red, and the GroEL domain with ADP bound is shown in blue.