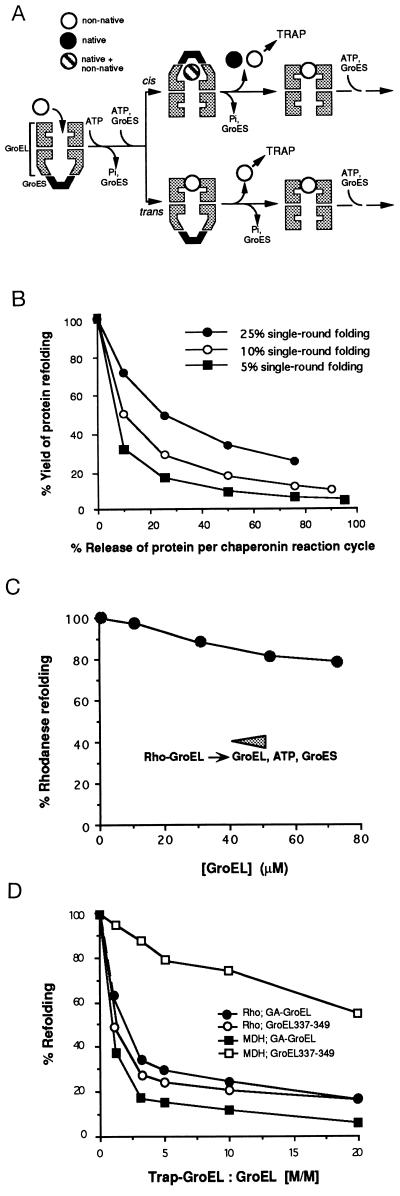
Figure 1.
Release of native and nonnative polypeptide during cycles of chaperonin-mediated protein folding. (A) A simplified model describing the basic chaperonin mechanism. GroEL is shown as a vertical section through the cylinder outlining the three-domain structure of the subunits. In dilute solution a fraction of bound polypeptide can be readily released from the GroEL cavity in a nonnative state from both the cis topology (GroES bound to the polypeptide-containing ring of GroEL) and the trans topology (GroES and polypeptide bound to opposite GroEL rings). This polypeptide may be captured by a noncycling mutant GroEL trap-GroEL before it can rebind to GroEL to undergo another folding trial. Trapping from the cis topology recently has been demonstrated (9, 10). (B) Relationship between yields of refolding and the rate of transfer of nonnative polypeptide to trap-GroEL. Yields are modeled assuming an increasing leakiness of GroEL for nonnative polypeptide and folding efficiencies per reaction cycle of 5%, 10%, and 25%. Note how the refolding yield would increase significantly at higher folding efficiencies as they are found for most other substrate proteins of GroEL studied (see refs. 11 and 12). The folding yield per cycle for rhodanese was experimentally determined to be ≈5%. The fraction of rhodanese that is transferred from wild-type GroEL to trap-GroEL per reaction cycle was measured in single-round transfer experiments (11) to be 25% and was constant at molar ratios of trap-GroEL to GroEL from 4- to 10-fold. (C) Rhodanese refolding in concentrated chaperonin solutions. The amount of active rhodanese obtained at 0.25 μM GroEL is set to 100%. (D) Effects of trap-GroEL on GroEL/GroES-mediated protein folding. Inhibition of folding of rhodanese (•, ○) and malate dehydrogenase (MDH) (▪, □) by GA-GroEL (•, ▪) and GroEL337/349 (○, □) at the indicated molar ratios of trap-GroEL over wild-type GroEL. Enzyme activities reached in the absence of trap-GroEL are set to 100%.