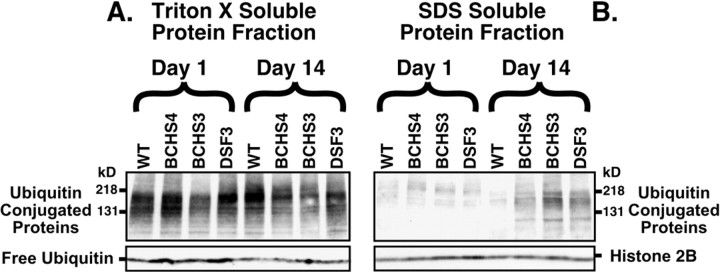
Fig. 6.
Immunoblot analysis of ubiquitin-conjugated proteins from adult Drosophila heads. Sequential protein extracts from heads of (1) wild-type, (2)bchs4/Df(2L)clot7, (3)bchs3/Df(2L)clot7, and (4) Df(2L)dsf3/Df(2L)clot7 flies were examined by Western blot analysis for free ubiquitin and conjugated forms of the protein. A, In the Triton X-100 fraction (readily soluble proteins) the levels of free ubiquitin and high molecular weight ubiquitin-conjugated proteins were not altered significantly for any genotype or age that was examined (1 or 14 d). Significant differences in less soluble ubiquitin-conjugated proteins also were not detected in young animals after more stringent extractions (SDS fraction). B, At 14 d there is a substantial increase in the amount of less soluble high molecular weight ubiquitinated conjugates in the SDS fraction for all three bchs mutant combinations, but not for wild-type controls. The bchs4 allele (2) has a nearly ninefold increase, whereas the Df(2L)dsf3/Df(2L)clot7 deletion genotype (4) has the highest level of accumulation (22.8-fold increase) when compared with wild-type controls. Results are summarized in Table 1.