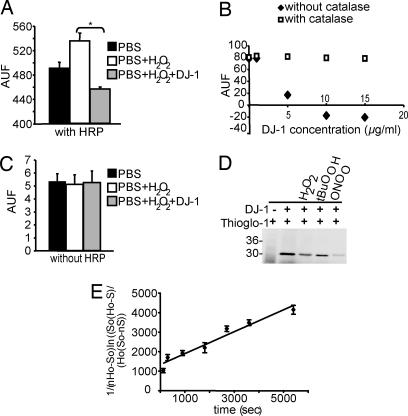
Fig. 3.
DJ-1 consumes H2O2. (A) Recombinant DJ-1 (5 μg/ml) incubated with 10 nM H2O2 reduced the H2O2 concentration in the reaction shown by the Amplex red assay. (B) Recombinant DJ-1 consumes H2O2 in a dose-dependent manner. This reaction is abolished by purified catalase. DJ-1 does not exhibit catalase-like activity as assessed by Clark electrode (data not show). (C) In the absence of HRP there is no signal, indicating that DJ-1 is not peroxidase-like. (D) Representative in-gel detection of cysteine residues labeled with Thioglo-1. Lane 1, Thioglo-1 without protein; lane 2, human DJ-1; lane 3, DJ-1 reacted with 250 μM hydrogen peroxide for 60 min at 37°C; lane 4, DJ-1 reacted with 250 and 500 μM t-butyl hydroperoxide for 60 min at 37°C; lane 5, DJ-1 reacted with 250 μM peroxynitrite for 5 min at 37°C. (E) Second-order rate constant determination for the reaction between 250 μM H2O2 and DJ-1 at 37°C using the times indicated. Catalase was added to remove excess H2O2, and samples were assayed for nonreactive cysteine by using Thioglo-1 labeling. Data represent the mean and SD from three different analyses. Significance was determined by ANOVA with the Student–Newman–Keuls test. *, P ≤ 0.05.