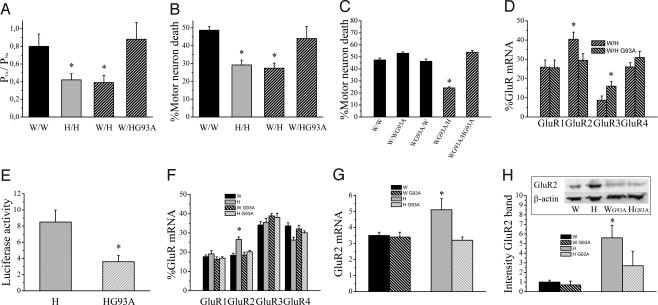
Fig. 5.
mt SOD1 in astrocytes abolishes their GluR2-regulating capacity. (A) PCa/PNa values of AMPA receptor currents in W/W, H/H, W/H, and W/H (n = 11–23; *, P < 0.03). (B) AMPA receptor-mediated excitotoxicity in W/W, H/H, W/H, and W/H (n = 5–18; *, P < 0.04). (C) AMPA receptor-mediated excitotoxicity in Wistar motor neurons with or without mt SOD1 grown on different combinations of astrocytes with or without mt SOD1 (n = 4–9; *, P ≤ 0.01, significantly different from all other groups). (D) Relative GluR mRNA expression in W/H and W/HG93A (n = 14–17; *, P < 0.04). (E) Effect of presence of mt SOD1 in Holtzman astrocytes on luciferase activity in cortical neurons seeded on Holtzman astrocytes (normalized to β-galactosidase activity and background activity of empty vector, n = 6–8; *, P = 0.015). (F) Relative GluR mRNA expression in the ventral spinal cord from Wistar and Holtzman rats with or without mt SOD1 determined by RT-PCR (n = 5–7; *, P ≤ 0.01, significantly different from other groups). (G) Real-time PCR for GluR2 normalized to 18S RNA with SYBR green on cDNA prepared from the ventral part of the spinal cord of Wistar and Holtzman rats with or without mt SOD1 (n = 8–10; *, P < 0.04, significantly different from other groups). (H) Western blot of GluR2 in the ventral part of the spinal cord from Wistar and Holtzman rats with or without mt SOD1 (n = 8–11; *, P < 0.05). Equal loading was demonstrated by β-actin staining, and the intensity of bands was normalized to the β-actin signal.