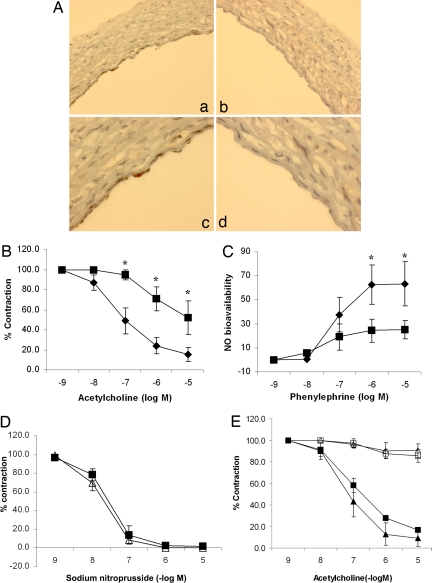
Fig. 1.
Endothelial SIRT1 regulates endothelium-dependent vasodilation and bioavailable NO through NOS. (A) A replication-deficient adenovirus encoding the myc-tagged catalytically inactive dominant negative mutant of SIRT1 (AdSIRT1(H363Y)) (a and c) was used to inhibit endogenous SIRT1 in the endothelium of rat aortic rings ex vivo. Control rings were infected with an adenovirus encoding the inert E. coli lacZ gene (AdLacZ) (b and d). Rings were fixed and immunostained with anti-myc antibody 24 h after adenoviral infection. Photomicrographs of representative rings at ×100 (a and b) and ×400 (c and d) are shown. (B) Endothelium-dependent vasodilation was determined by measuring relaxation of rings preconstricted with phenylephrine to the vasodilator acetylcholine. AdSIRT1(H363Y) (■) and AdLacZ (◆) are shown. *, P < 0.001 compared with AdLacZ (n = 7). (C) Bioavailable NO was determined by measuring the difference in phenylephrine-induced vasoconstriction in the presence and absence of the NOS inhibitor l-NAME. AdSIRT1(H363Y) (■) and AdLacZ (◆) are shown. *, P < 0.01 compared with AdSIRT1(H363Y) (n = 7). (D) Endothelium-independent vasodilation was determined by measuring relaxation of rings preconstricted with phenylephrine to the NO donor sodium nitroprusside. AdSIRT1(H363Y) (△) and AdLacZ (■) (n = 4) are shown. (E) Rat aortic rings were infected ex vivo with AdLacZ (■ and □) or AdSIRT1 (△ and ▲). AdSIRT1 encodes wild-type SIRT1. Endothelium-dependent vasodilation, in the presence (□ and △) and absence (■ and ▲) of the NOS inhibitor l-NAME, was determined by measuring relaxation of rings preconstricted with phenylephrine, to the vasodilator acetylcholine. n = 4 rings in each group.