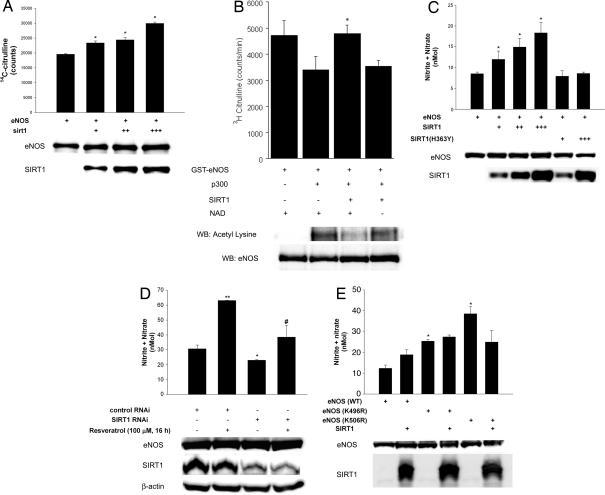
Fig. 3.
SIRT1 stimulates eNOS activity and increases endothelial NO. (A) NOS catalytic activity (conversion of arginine to citrulline) in lysates of COS7 cells expressing eNOS, with and without SIRT1 overexpression, was measured. Lysates were immunoblotted with anti-SIRT1 and anti-eNOS antibodies. *, P < 0.05 compared with eNOS without SIRT1. (B) NOS catalytic activity in vitro, in the absence and presence of p300, SIRT1, and NAD. Total and acetyllysine eNOS is shown at bottom. *, P < 0.05 compared with eNOS + p300. (C) Metabolites of NO (nitrite and nitrate) were measured in media of COS7 cells expressing eNOS, with and without overexpression of SIRT1, or inactive SIRT1 (H363Y). Whole-cell lysates were immunoblotted with anti-eNOS and anti-SIRT1 antibodies. Endogenous SIRT1 is not seen in the lysates because of underexposure of the blots. *, P < 0.05 compared with eNOS without SIRT1. (D) Nitrite and nitrate were measured in media of rat aortic endothelial cells that were transfected with SIRT1 RNAi or control RNAi, or treated with the SIRT1 activator resveratrol. *, P < 0.05 compared with control RNAi. **, P < 0.01 compared with control RNAi. #, P > 0.05 compared with SIRT1 RNAi. (E) Nitrite and nitrate were measured in COS7 cells expressing eNOS (WT), eNOS (K496R), or eNOS(K506R), with and without SIRT1 overexpression. *, P < 0.05 compared with eNOS (WT).