Abstract
The peptide hormone hepcidin is the principal regulator of systemic iron homeostasis. We examined the pathway by which iron stimulates the production of hepcidin. In humans who ingested 65 mg of iron, the increase in transferrin saturation preceded by hours the increase in urinary hepcidin excretion. Increases in urinary hepcidin concentrations were proportional to the increment in transferrin saturation. Paradoxically, in previous studies in primary hepatocytes and cell lines, hepcidin response to iron or iron transferrin was not observed. We now report that freshly isolated murine primary hepatocytes responded to holotransferrin but not apotransferrin by increasing hepcidin mRNA. Hepcidin increase was not due to contamination of the transferrin preparations by endotoxin, a potent pathologic stimulus of hepcidin synthesis. Using this culture system, we showed that holotransferrin concentrations regulate hepcidin mRNA concentrations through a hemojuvelin/BMP2/4–dependent pathway. Although BMP9 is known to be expressed in the liver and potently increased the basal concentrations of hepcidin mRNA, it did not interact with hemojuvelin, and interference with its signaling pathway did not affect iron regulation. Fresh primary hepatocytes constitute a sufficient system for the regulation of hepcidin by physiologic iron stimuli and will greatly facilitate studies of major disorders of iron homeostasis.
Introduction
Hepcidin (HAMP) is the principal iron-regulatory hormone.1 It is predominantly produced in the liver, circulates in blood, and is excreted in urine. Hepcidin regulates systemic iron homeostasis by inhibiting dietary iron absorption in the small intestine, recycling of iron from senescent erythrocytes by macrophages, and iron mobilization from hepatic stores
Hepcidin production is affected by dietary or parenteral iron loading, iron stores, erythropoietic activity, tissue hypoxia, and inflammation.2–5 In healthy humans and mice, iron loading by ingestion or injection induces hepcidin synthesis. However, how iron regulates hepcidin production is still unknown. Previous in vitro studies with hepatoma cell lines and primary hepatocytes reproduced the response of hepcidin during inflammation and hypoxia but failed to demonstrate increased hepcidin synthesis in response to iron loading. It appeared that some essential regulatory components present in vivo were missing in isolated hepatocytes.
In hereditary hemochromatosis, dietary iron is hyperabsorbed and accumulates in tissues, eventually causing organ damage. Hepcidin analyses in human subjects and in animal models indicate that most hereditary hemochromatosis is due to hepcidin deficiency resulting from primary mutations in human hemochromatosis gene (HFE), transferrin receptor 2 (TFR2), the juvenile hemochromatosis gene hemojuvelin (HJV), or the hepcidin gene itself. This implies that HFE, transferrin receptor 2, and hemojuvelin play important roles in the regulation of hepcidin. Mutations of hemojuvelin result in the most severe form of hereditary hemochromatosis, which is phenotypically indistinguishable from the juvenile hemochromatosis caused by mutations in the hepcidin gene itself. Using an antisense strategy in a hepatoma cell line, we suppressed the expression of hemojuvelin and observed that hepcidin expression correlated with hemojuvelin expression. Hemojuvelin protein exists both as a cell-associated GPI-linked form and a soluble form that can be detected in cell-culture supernatant as well as in human6 and rat7 blood. The amount of soluble hemojuvelin in the cell-culture supernatant was reduced by treatment with iron or holotransferrin. Soluble hemojuvelin suppressed hepcidin production in primary hepatocytes in a dose-dependent manner,6 suggesting that hemojuvelin is a possible mediator of the response to iron loading.
Recent studies implicated bone morphogenetic protein (BMP) signaling in the regulation of hepcidin synthesis.8 BMPs are a subgroup of the transforming growth factor β (TGF-β) superfamily involved in a variety of biologic processes.9,10 The BMP signaling pathway is activated by dimeric ligands bringing type I and type II receptor serine/threonine kinases to close proximity on the cell surface. The constitutively active type II receptor kinase phosphorylates and activates the kinase activity of type I receptor, which in turn relays the signal onto the receptor-regulated Smad (R-Smad) by phosphorylating Smad 1, 5, and 8. Upon phosphorylation, R-Smad proteins form a complex with the common mediator Smad4 (Co-Smad). The activated Smad complex translocates into the nucleus and regulates transcription of its target genes. The specificity of target gene activation is subject to combinatorial regulation by the Smad complex and other nuclear cofactors. Mice with liver-specific Smad4 ablation produce very little hepcidin mRNA and develop hemochromatosis, indicating that this transcription factor is required for hepcidin synthesis. In these mice, hepatic hepcidin mRNA concentrations are not increased in response to either iron or inflammatory stimuli, and it was suggested that Smad4 may have a chromatin-opening effect on hepcidin.11
The BMP signaling pathways are regulated by a variety of modulators including inhibitory Smads (Smad 6 and 7), ligand traps (such as Noggin, a potent antagonist of several BMP ligands), and BMP coreceptors, which enhance BMP ligands binding to type I and II receptors.12 Babitt et al8 showed that overexpression of hemojuvelin in the hepatoma cell line HepG2 enhanced the expression of a BMP-responsive reporter and increased hepcidin mRNA concentrations. Direct binding between BMP2 and a soluble hemojuvelin-Fc fusion protein was also observed, suggesting that hemojuvelin is a BMP coreceptor.8 However, the involvement of BMP signaling in hepcidin induction by iron has not been demonstrated.
The lack of in vitro systems that reconstitute hepcidin induction by iron remains a major obstacle to the analysis of the regulation of hepcidin by iron and the dysregulation of this process in iron disorders. In vivo, it is difficult to dissect potential iron-regulatory pathways with various inhibitors without complications from signals generated outside of the target tissue. In this report, we describe a primary mouse hepatocyte cell culture system that consistently responds to holotransferrin treatment. Using this system, we demonstrate that induction of hepcidin synthesis by holotransferrin is mediated by a hemojuvelin/BMP2/4–dependent pathway but is independent of the BMP9 pathway.
Patients, materials, and methods
Iron supplementation in humans
The study was approved by the Human Subjects Protection Committee at UCLA. Informed consent was obtained from all human subjects in accordance with the Declaration of Helsinki. On the first morning, 6 healthy volunteers ingested 65 mg of iron as ferrous sulfate (Nature Made, Mission Hills, CA). Serum and urine samples were obtained at 0, 5, 10, 24, 36, and 48 hours after iron ingestion.
Urinary hepcidin assay
Urinary hepcidin concentrations were determined as described previously.13 Briefly, cationic peptides were extracted from urine using ion-exchange chromatography (CM Macro-prep; BioRad Laboratories, Richmond, CA), lyophilized, and resuspended in 0.01% acetic acid. Urinary creatinine concentrations were measured by UCLA Clinical Laboratories. Urine extracts equivalent to 0.1 to 0.5 mg of creatinine were analyzed by immunodot assay using rabbit anti–human hepcidin antibody.14 Dot blots were quantified using Quantity One software (BioRad Laboratories), and urinary hepcidin concentrations were expressed as ng hepcidin per mg creatinine. For each of the 6 subjects, hepcidin concentrations at different time points were normalized to the 0-hour value to measure fold change in response to iron ingestion.
Serum iron measurement
Serum transferrin saturation was determined as described previously.13 Briefly, 50 μL of serum was assayed for serum iron and the unsaturated iron-binding capacity (UIBC) was determined with a colorimetric method (Diagnostic Chemicals, Oxford, CT). The total iron-binding capacity (TIBC) was calculated as the sum of serum iron and UIBC, and the percentage of transferrin saturation as serum iron/TIBC × 100.
Reagents and mice
Human apotransferrin and holotransferrin were from Millipore (Billerica, MA). Recombinant human BMP-2, recombinant human BMP-4, recombinant human BMP-9, recombinant mouse ALK1/Fc chimera, monoclonal antihuman BMP-2/4 antibody, and recombinant mouse noggin were from R&D systems (Minneapolis, MN). E-TOXATE LPS endotoxin standard and E-TOXATE Kit for Limulus Amebocyte Lysate test were from Sigma-Aldrich (St Louis, MO).
C57BL/6J mice were from Charles River Laboratories (Wilmington, MA). MyD88-deficient mice15,16 on a C57BL/6J genetic background were gifts from Dr Genhong Cheng (UCLA, Los Angeles, CA), who originally obtained them from Dr Shizuo Akira (Osaka University, Osaka, Japan). All mice were maintained on an NIH 31 rodent diet (iron content 336 mg/kg; Harlan Teklad, Indianapolis, IN)
Production and purification of mouse soluble hemojuvelin
To express mouse soluble hemojuvelin (msHJV), a mouse hemojuvelin cDNA (truncated by 279 nucleotides [nt's] at the 3′ end with an added stop codon) was cloned into a lentiviral transfer vector pRRL-sin-hCMV-MCS(R)-pre-cPPT. Lentiviral particles were packaged by the UCLA Vector Core Facility. A permanent cell line expressing mouse soluble hemojuvelin was generated by transducing the HEK293 cell line with lentivirus overnight. This cell line was maintained in an equal volume mixture of Dulbecco Modified Eagle Medium (DMEM; Invitrogen, Carlsbad, CA) and Pro-293a-CDM (Cambrex, Brussels, Belgium) and supplemented with 5% fetal bovine serum. Soluble hemojuvelin Gln33-Arg328 was purified from conditioned culture medium by cation exchange chromatography (CM Prep; BioRad), followed by high-performance liquid chromatography on a C4 reverse-phase column (Vydac, Deerfield, IL; 214TP54) eluted with an acetonitrile gradient. The apparent size of this protein was the same as the size of mouse soluble hemojuvelin released from cells transfected with a full-length mouse hemojuvelin construct (L.L., E.N., J.B.G., Dharma Thapa, V. G. and T.G., in preparation).
Mouse primary hepatocyte isolation
Mouse primary hepatocytes were isolated using a Krebs-Henseleit solution/collagenase perfusion protocol according to Amaxa Biosystems (Amaxa, Gaithersburg, MD). The viable hepatocyte population was further purified by a Percoll gradient centrifugation.17 Hepatocytes were plated in collagen-coated plates at 3 × 105 cells per well in a 6-well plate or 1.5 × 105 cells per well in a 12-well plate in William E medium supplemented with 5% fetal bovine serum and antibiotics. Cells were allowed to attach for 2 hours before switching to fresh medium and adding all treatments. All cells were treated for 18 to 24 hours before collection in TRIzol (Invitrogen).
RNA isolation and qRT-PCR assay
RNA from primary mouse hepatocyte cultures was prepared using TRIzol (Invitrogen) according to the manufacturer's instructions. Single-pass cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). The quantitative real-time polymerase chain reaction (qRT-PCR) was performed using iQ SYBR Green Supermix (Bio-Rad). Murine hepcidin 1 (Hepc1) mRNA concentrations were normalized to murine β-actin. The following primers were used in qRT-PCR: murine hepcidin1: forward, 5′-TTGCGATACCAATGCAGAAGA-3′; reverse, 5′-GATGTGGCTCTAGGCTATGTT-3′18; murine β-actin: forward, 5′-ACCCACACTGTGCCCATCTA-3′; reverse, 5′-CACGCTCGGTCAGGATCTTC-3′.
As described by Pfaffl,19 we first normalized mRNA concentrations of the target gene (Hepc1) to a reference stable housekeeping gene (β-actin) and then presented the measurement as a ratio to the control treatment within each experiment. The average relative expression value of the triplicate control treatments was assigned as 1 in each experiment.
Results
Acute iron load in human subjects elicits hepcidin release proportional to peak transferrin saturation
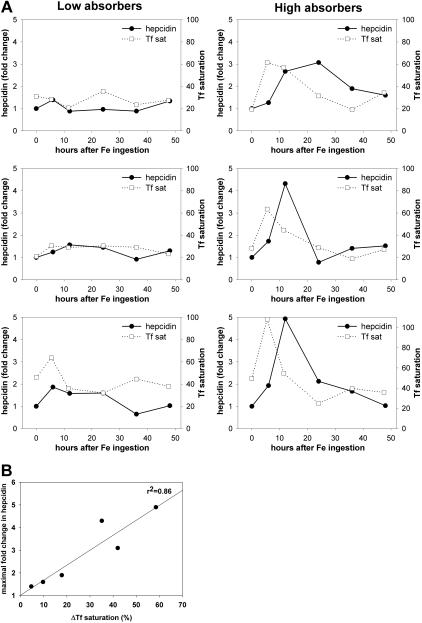
Simulating a conventional iron replacement regimen, at 8 am on the test day, nonfasting human volunteers (n = 6) ingested 65 mg iron as a ferrous sulfate tablet. Transferrin saturation and urinary hepcidin/creatinine ratio were measured immediately before and at 5, 10, 24, 36, and 48 hours after ingestion. Expected variations in iron absorption resulted in differential increases in transferrin saturation at 5 hours and a subsequent decline (Figure 1A). In 3 subjects with a more than 2-fold increase in urinary hepcidin concentrations, the peak hepcidin concentrations were seen at 12 to 24 hours. The peak hepcidin fold-increase correlated with the difference between baseline and peak transferrin saturation (Figure 1B; Pearson correlation coefficient r = 0.93, P = .007, n = 6). These observations suggest that hepcidin synthesis is stimulated by acute iron loading and that the iron load may be sensed by the hepcidin-producing hepatocytes through changes in transferrin saturation and the resulting change in holotransferrin concentration.
Figure 1.
Hepcidin response to dietary iron is proportional to the increase in transferrin (Tf) saturation. Six subjects ingested 65 mg of iron (as ferrous sulfate) and their urinary hepcidin concentrations and Tf saturation were assayed at 0, 5, 10, 24, 36, and 48 hours after ingestion. (A) Each graph depicts the response of a single subject. Urinary hepcidin, expressed as the fold change in comparison to 0-hour levels, is shown as the solid line; percentage Tf saturation is shown as the dashed line. According to the increase in transferrin saturation, which reflects the rate of iron absorption, the subjects were grouped into “low absorbers” and “high absorbers.” (B) Correlation between the increase in urinary hepcidin and the increase in transferrin saturation after iron ingestion. The solid line represents linear regression between the maximal hepcidin increase (expressed as fold change in comparison to 0-h levels) and the maximal transferrin saturation increase (expressed as difference in comparison to 0-h levels, ΔTf saturation; r2 = 0.86, P = .007).
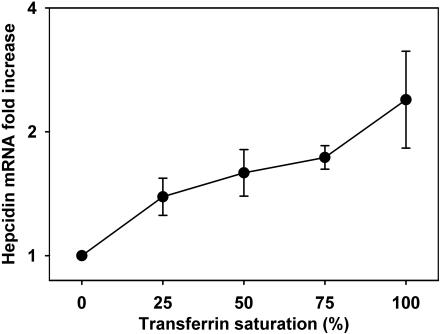
Freshly prepared primary hepatocyte cultures respond to transferrin saturation by a dose-dependent increase in hepcidin mRNA
Next we determined the primary hepatocyte response to increasing transferrin saturation at a constant total transferrin concentration of 30 μM. We prepared a 0% to 100% range of transferrin saturation by mixing different proportions of apotransferrin and holotransferrin. Primary hepatocyte cultures were treated 2 hours after plating and collected for mRNA analysis after 18 to 24 hours incubation. Hepcidin mRNA concentration showed a steady increase when treated with increasingly iron-saturated transferrin (Figure 2). In the presence of 100% iron-saturated holotransferrin, hepcidin mRNA expression was induced 2.5 ± 0.6-fold in comparison to iron-free transferrin treatment (30 μM apotransferrin alone; P = .008 by Mann-Whitney test, n = 5 mice). A non–transferrin-bound iron form, 60 μM ferric ammonium citrate, showed no effect on hepcidin expression (data not shown), indicating that the induction of hepcidin expression is in response to transferrin saturation but not to elemental iron or elevated cellular iron level.
Figure 2.
Dose dependence of hepcidin mRNA expression in primary mouse hepatocyte cultures exposed to different transferrin saturations. Primary mouse hepatocytes were isolated from wild-type C57BL/6J mice (n = 5 mice). Mixtures of apotransferrin and holotransferrin were added to hepatocyte cultures at a total transferrin concentration of 30 μM. Hepcidin mRNA concentration at each transferrin saturation level was plotted as fold increase in comparison to 30 μM apotransferrin treatment alone (0% transferrin saturation). Means and standard deviations are shown. Increasing transferrin saturations resulted in progressive increase in hepcidin mRNA in primary hepatocytes.
An extended culture period before addition of transferrin treatment resulted in a gradual decrease in the induction of hepcidin mRNA concentration, in response to holotransferrin treatment. Primary hepatocyte cultures showed no response to 30 μM holotransferrin when treated 48 hours after plating (data not shown).
Increase in hepcidin production in primary hepatocyte cultures is not due to endotoxin contamination in human apotransferrin and holotransferrin
Hepcidin mRNA is potently induced by lipopolysaccharide (LPS), as well as by IL-6 and other cytokines.2,14 We therefore examined the possibility that the holotransferrin increases hepcidin mRNA due to low-level contamination with endotoxin. First, we used the Limulus Amebocyte Lysate (LAL) test to estimate the endotoxin content of human apotransferrin and holotransferrin. Endotoxin was undetectable (less than 0.06 endotoxin units [EU]/mg) in both apotransferrin and holotransferrin. Thus, the 30-μM concentration of apotransferrin/holotransferrin, used to treat mouse hepatocytes, contained less than 0.14 EU/mL endotoxin. Based on this estimate, we chose to use 0.25 EU/mL LPS endotoxin standard as a baseline endotoxin-negative control and a higher concentration of 100 EU/mL (equivalent to approximately 100 ng/mL LPS) as a positive control for the inflammatory response to endotoxin in primary hepatocyte cultures.
To examine further whether holotransferrin could induce hepcidin through an endotoxin-like contaminant, we tested the iron response in hepatocytes from MyD88-deficient mice. MyD88 is an adaptor protein common to toll-like receptor (TLR) signaling pathways, including the TLR4 signaling pathway. MyD88-deficient mice have greatly decreased or absent LPS responses, such as LPS-induced endotoxin shock and production of serum inflammatory cytokines (IL-6, TNFα, and IL-1β).15,16
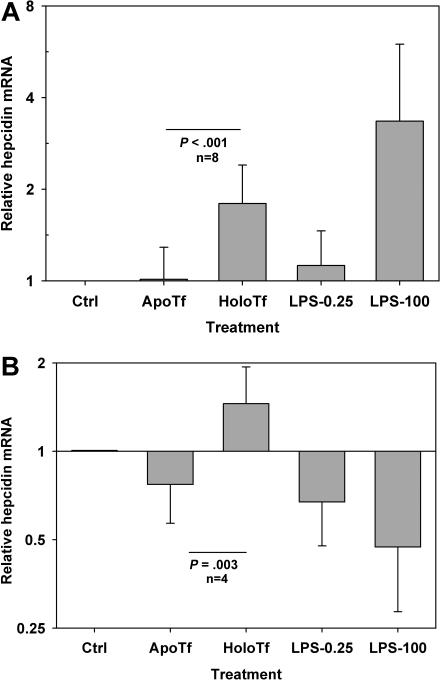
Primary hepatocyte cultures isolated from either wild-type mice or MyD88-deficient mice responded to 30-μM holotransferrin treatment by inducing hepcidin mRNA to a similar extent (Figure 3). The average induction was 1.8-fold in wild-type mice (P < .001 by 1-way repeated measures [RM] analysis of variance [RMANOVA] test comparing cells treated with solvent, apotransferrin, holotransferrin, or low-dose LPS; n = 8 mice; Figure 3A) and 2-fold in MyD88-deficient mice (P = .003 by 1-way RM ANOVA test; n = 4 mice; Figure 3B). Whereas 100 EU/mL LPS induced hepcidin production by an average of 3.3-fold in wild-type hepatocytes, this effect was not observed in MyD88-deficient hepatocytes. On the contrary, 100 EU/mL LPS led to an approximately 2-fold suppression of hepcidin mRNA compared with control treatment (1-tail paired t test, P = .03). LPS at 0.25 EU/mL did not affect hepcidin mRNA concentration in either hepatocyte culture.
Figure 3.
Holotransferrin specifically induces hepcidin mRNA expression in mouse primary hepatocytes. Primary hepatocytes were isolated from both wild-type (A) and MyD88-deficient mice (B) on C57BL/6J background. Hepatocyte cultures were treated with 30 μM apotransferrin or holotransferrin or with 0.25 EU/mL or 100 EU/mL LPS endotoxin standard. Means and standard deviations are shown. (A) In wild-type hepatocytes (n = 8 mice), only holotransferrin and 100 EU/LPS induced hepcidin mRNA expression (P < .001). (B) In MyD88-deficient hepatocytes (n = 4 mice), only holotransferrin induced hepcidin mRNA expression (P = .003) and LPS suppressed it (P = .03).
These data indicate that the increased hepcidin production after holotransferrin treatment is not due to endotoxin contamination of the holotransferrin preparation.
Distinct effects of mouse soluble hemojuvelin on BMP2, 4, and 9 induction of hepcidin production
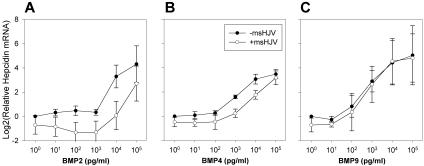
A previous study by Babitt et al8 suggested that hemojuvelin functions as a BMP coreceptor by binding to BMP2/4, which in turn facilitates BMP signaling and stimulates hepcidin production. Later, Truksa et al20 showed that not only BMP2 and 4 but also BMP9 are potent inducers of hepcidin production. To investigate the role of hemojuvelin in BMP signaling, we used mouse soluble hemojuvelin (msHJV) as a specific inhibitor of the hemojuvelin signaling pathway6 and measured hepcidin mRNA concentrations in primary hepatocytes treated with a combination of 10 μg/mL msHJV and a range of BMP2, 4, or 9 concentrations (0.01-100 ng/mL).
Simultaneous addition of msHJV decreased the induction of hepcidin by BMP2/4. The suppression of hepcidin mRNA level by msHJV was dependent on the concentration of BMP2 ligand, with the largest suppressive effect at 10 ng/mL of BMP2 (Figure 4A). BMP4 treatment showed a similar pattern (Figure 4B). These results are consistent with previous studies showing the binding of hemojuvelin to BMP2/48 and support the hypothesis that soluble hemojuvelin acts as decoy, competing with the GPI-hemojuvelin/BMP receptor complex for the BMP2/4 ligands.
Figure 4.
Distinct effects of mouse soluble hemojuvelin on BMP2, 4, and 9 induction of hepcidin production. Primary mouse hepatocytes were isolated from wild-type C57BL/6J mice. Hepatocyte cultures were treated with a combination of either 10 μg/mL msHJV (○) or its solvent (●) with 10-fold serial dilutions of (A) BMP2, (B) BMP4, or (C) BMP9 ranging from 0.01 to 100 ng/mL. Means and standard deviations are shown.
For BMP9, the suppressive effect of 10 μg/mL msHJV on hepcidin mRNA was much smaller and disappeared progressively with increasing concentrations of BMP9 (Figure 4C), suggesting that BMP9 stimulates hepcidin mRNA expression through a hemojuvelin-independent pathway. The small suppression of hepcidin mRNA observed at low concentrations of BMP9 could be due to low amounts of BMP2/4 already present in the hepatocyte cultures.
Differential effects of the inhibition of BMP2/4 and BMP9 signaling pathways on hepcidin induction by holotransferrin
Noggin is a highly conserved BMP antagonist with a high binding affinity for BMP2/4/7 and growth differentiation factor-5 (GDF-5). Noggin has been shown to block the BMP epitopes that bind to type I and type II receptors and thus inhibits BMP signaling.21,22
ALK1 (activin receptor-like kinase 1) is a type I receptor of the TGF-β receptor family predominantly expressed in endothelial cells and other highly vascularized tissues. It has been described as an orphan BMP receptor. Recent studies indicate that BMP9 is a potent ALK1 ligand through ALK1 interactions with type II receptors BMPRII or ActRIIA. Recombinant soluble ALK1/Fc chimera protein, which includes the extracellular domain of ALK1, specifically abolishes BMP9 response in endothelial cells.23 We confirmed that, as expected, ALK1/Fc suppressed hepcidin induction by BMP9 but not BMP2 in mouse primary hepatocytes (data not shown).
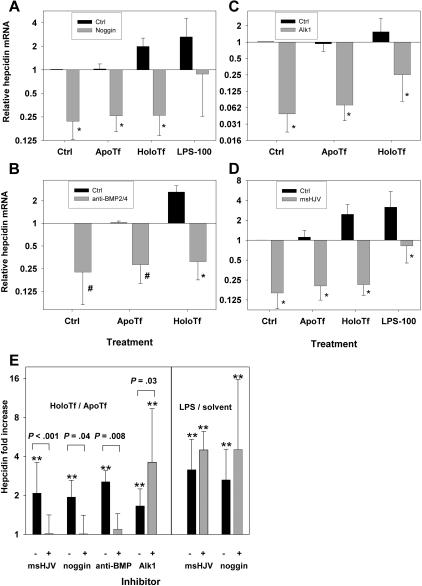
To investigate whether holotransferrin stimulates hepcidin production by BMP signaling, we used noggin and a neutralizing anti-BMP2/4 antibody to inhibit the BMP2/4 signaling pathway and a recombinant soluble mouse ALK1/Fc chimera protein to inhibit the BMP9 signaling pathway. Treatment with noggin (1 μg/mL) alone significantly suppressed hepcidin production in primary hepatocyte cultures an average of 4-fold (Figure 5A). In addition to reduction in baseline hepcidin expression, the response of hepatocytes to holotransferrin was greatly diminished (P = .04, paired t test, n = 5 mice; Figure 5E left panel). Treatment of hepatocytes by a neutralizing antibody to BMP2/4 (20 μg/mL) produced similar results as noggin treatment (Figure 5B), abolishing hepcidin induction by holotransferrin (P = .008, paired t test, n = 5 mice; Figure 5E left panel), confirming the specificity of noggin for BMP2/4 in this setting. Therefore, our results suggest that holotransferrin induces hepcidin production in primary hepatocyte cultures through a BMP2/4-dependent pathway. In contrast, the LPS stimulation of hepcidin production was intact in the presence of noggin (Figure 5E right panel) or BMP2/4 antibody (not shown). LPS induction of hepcidin expression is mediated by toll-like receptors and, secondarily, by inflammatory cytokines. Our results suggest that these pathways regulate hepcidin independently of the BMP pathway.
Figure 5.
Effects of noggin, anti-BMP2/4 antibody, ALK1/Fc, and msHJV on hepcidin expression and response to holotransferrin treatment. Primary mouse hepatocytes from wild-type C57BL/6J mice were treated with a combination of (A) 1 μg/mL noggin plus 30 μM apoTf/holoTf or 100 EU/mL LPS (n = 5 mice); (B) 20 μg/mL anti-BMP2/4 antibody plus 30 μM apoTf/holoTf (n = 5 mice); (C) 500 ng/mL ALK1/Fc plus 30 μM apoTf/holoTf (n = 7 mice); (D) 30 μg/mL msHJV plus 30 μM apoTf/holoTf or 100 EU/mL LPS (n = 5 mice). (E) Comparison of the effects of msHJV, noggin, anti-BMP2/4 antibody, and ALK1/Fc on the induction of hepcidin by holotransferrin versus apotransferrin (left panel) and LPS versus solvent control (right panel). Statistics are as follows: *P ≤ .005; #P < .03, paired t test, comparing (A) noggin treatment with control, (B) anti-BMP2/4 antibody treatment with control, (C) ALK1/Fc treatment with control, (D) msHJV treatment with control; **P < .005, 1-way RM ANOVA, comparing apotransferrin, holotransferrin, and LPS treatments to respective solvent controls (E). Means and standard deviations are shown.
Recombinant mouse ALK1/Fc chimera (500 ng/mL) treatment by itself also significantly suppressed hepcidin production in primary hepatocyte cultures an average of 16-fold (Figure 5C). However, ALK1/Fc treatment did not inhibit the hepcidin response to holotransferrin (Figure 5E left panel). Interestingly, although the baseline hepcidin mRNA level was reduced at least as much as in noggin-treated cultures (Figure 5A), the holotransferrin induction of hepcidin expression was enhanced to an average of 4-fold compared with an approximately 1.7-fold induction in the absence of ALK1/Fc (P = .03, paired t test, n = 7 mice; Figure 5E left panel). Similarly, ALK1/Fc treatment of MyD88-deficient hepatocytes did not interfere with holotransferrin stimulation of hepcidin expression (data not shown). These results indicate that holotransferrin stimulates the iron-sensing pathway in a BMP9-independent manner.
Mouse soluble hemojuvelin inhibits the induction of hepcidin mRNA by holotransferrin in primary hepatocyte cultures
Next we tested the effect of msHJV as a specific inhibitor of hemojuvelin on holotransferrin induction of hepcidin mRNA expression in primary hepatocyte cultures. The baseline hepcidin production was suppressed by an average of 6-fold in primary hepatocyte cultures treated with 30 μg/mL msHJV (Figure 5D). Like 1 μg/mL noggin and 20 μg/mL anti-BMP2/4 antibody treatments, 30 μg/mL msHJV completely blocked the induction of hepcidin mRNA in holotransferrin-treated hepatocytes (P < .001, paired t test, n = 5 mice; Figure 5E left panel), whereas LPS stimulation of hepcidin production was intact (Figure 5E right panel). Thus, the effect of soluble hemojuvelin on hepcidin induction by iron resembles the effect of blocking the BMP2/4 signaling. Both were sufficient to abolish hepcidin mRNA induction in response to iron loading in hepatocytes. In contrast, inhibition of BMP9 signaling did not interfere with the holotransferrin-mediated induction of hepcidin mRNA.
In summary, our results suggest that hemojuvelin mediates holotransferrin-induced hepcidin mRNA expression solely through BMP2/4 signaling. BMP9 regulates baseline hepcidin expression but is not involved in the induction of hepcidin mRNA by holotransferrin.
Discussion
Inducibility of hepcidin mRNA by oral or parenteral iron loading was the first indication of the role of hepcidin in iron homeostasis.2 Iron-dextran injection induced hepcidin-1 mRNA by 2.5-fold within 1 week, and this effect was sustained at the same level (2-6 fold) for at least 2 months.2 A similar result was also obtained by placing mice on a diet containing 3% carbonyl iron for 2 months.2,4 At the other extreme, an iron-deficient diet caused a dramatic drop of hepcidin mRNA expression in rats within 2 weeks.3 Mice maintained on an iron-deficient diet (2-4 mg iron/kg) for 2 weeks followed by a standard diet (336 mg iron/kg) for 24 hours showed an average of approximately 10-fold induction in hepatic hepcidin mRNA.13 In humans, urine immunoassay for hepcidin measured this response at the peptide level: the ingestion of a single iron tablet (65 mg Fe as ferrous sulfate) increased urinary hepcidin within 24 hours, with an average of 5.4-fold induction of urinary hepcidin excretion.13 In the current study (Figure 1) we showed that hepcidin excretion after an acute iron load was proportional to the rise in transferrin saturation.
The mechanism by which iron regulates hepcidin is still largely unknown. The severe systemic iron overload in transferrin-deficient humans and mice suggests that the feedback regulation of iron absorption, and, by implication, the feedback regulation of hepcidin synthesis, may depend predominantly on sensing the concentration of iron bound to transferrin.24,25 The likely role of transferrin-bound iron in iron sensing is further supported by the relatively high hepcidin mRNA level in the livers of mice that are anemic but have high transferrin saturations because of impaired utilization of iron transferrin for hemoglobin synthesis.5,26 In addition, hepcidin is deficient in humans and mice with homozygous disruption of transferrin receptor 2,13,27 and the stability of this receptor is regulated by holotransferrin in the physiologic range of concentration.28,29 Of the 3 forms of iron transferrin (2 monoferric transferrins and differic holotransferrin), holotransferrin would be expected to have a predominant role in iron sensing, as it is the only form whose concentration increases monotonically with transferrin saturation.30
A major obstacle to examining the sensing of holotransferrin, and the specific pathways by which it may induce hepcidin, has been the lack of an in vitro system to reproduce the in vivo induction of hepcidin by iron loading.2,14,31 In this report, our primary hepatocyte culture system showed a consistent increase of hepcidin mRNA concentration upon treatment with holotransferrin, with an average of approximately 2-fold increase in response to 30-μM diferric holotransferrin treatment (Figure 2). This is comparable to the range of hepcidin induction in previous reports using in vivo models. We also established that the response to iron was holotransferrin specific and was not observed with non–transferrin-bound iron and not caused by LPS contamination in the apotransferrin and holotransferrin preparations.
Holotransferrin induction of hepcidin mRNA was dose dependent. The normal range of total serum transferrin is 30 to 60 μM in humans28 and 40 to 80 μM in rats.7 Basal transferrin saturation in a healthy adult human averages at around 30%, with a range from 15% to nearly 50%. In mice, the transferrin saturation is higher, ranging from 60% to 80%,32 perhaps as a result of the very high iron content of the standard mouse diets. Transferrin saturation may chronically reach 100% during iron overload in pathologic states. Acutely, a single oral dose of 100 mg iron in the form of ferrous ascorbate was reported to increase transferrin saturation from 18% (basal level) to approximately 60%.33 Our own measurements indicate that even higher transferrin saturations can be reached with the first dose of conventional iron replacement therapy (Figure 1). An iron-deficient diet (less than 2 mg Fe/kg) can lower transferrin saturation in weaning male rats to less than 5% within 2 to 3 weeks compared with 60% to 80% in a control group.7 Relevant to iron sensing by hepatocytes, transferrin saturation in the portal circulation is believed to be higher, due to the first-pass effect of dietary iron absorbed through intestine and entering the liver through the portal vein. These numbers suggest that variations in iron absorption and consumption under normal and pathologic conditions should cause large variations in serum transferrin saturation that would be reflected in the concentrations of holotransferrin sensed by hepatocytes. Thus we appropriately modeled these changes in vitro by exposing hepatocytes to 30 μM holotransferrin or apotransferrin.
In previous reports, conventional primary hepatocyte cultures did not show increased hepcidin expression after iron loading.2,14,27 Unlike conventional cultures that are usually allowed to recover for 1 to 3 days before holotransferrin treatment, the current study used fresh primary hepatocyte cultures within hours of harvest. We confirmed that primary hepatocytes cultured for 48 hours before treatment with 30 μM holotransferrin no longer responded by increasing hepcidin mRNA. It has been reported that hepatocytes lose the expression of a number of liver-specific genes over time when maintained for a few days without differentiation-promoting factors.2 This prolonged culture period could result in severe loss of essential components of the iron-sensing and signaling machinery. The early treatment likely preserves the iron-sensing apparatus and the responsiveness to holotransferrin.
Even freshly isolated primary hepatocytes may not fully represent the intact iron-sensing machinery for regulating hepcidin. Upon initial plating in William E medium supplemented with 5% fetal bovine serum, primary hepatocytes rapidly lose approximately 95% of hepcidin mRNA transcripts within the initial 8 hours of culture, and this is followed by a plateau for at least 72 hours afterward (data not shown). Frequent changes of media (twice per hour) during the initial 6-hour incubation did not change hepcidin mRNA levels (data not shown), making it unlikely that hepcidin mRNA was suppressed by a secreted factor from the primary hepatocyte culture. While the half-life of hepcidin mRNA (determined by actinomycin-D treatment in a hepatoma cell line, Hep3B, using β-actin as a stable mRNA reference) is approximately 6 hours (J.B.G., L.L., unpublished results, December 2006), the loss of hepcidin mRNA in primary hepatocytes during the initial culture showed an apparent half-life of approximately 1.6 hours. Hepcidin mRNA may be rapidly degraded due to transient hypoxia or the loss of a membrane component susceptible to collagenase digestion during the liver perfusion and hepatocyte isolation. The plateau phase of the hepcidin mRNA level after an initial 8 hours of culture could indicate the partial recovery of this membrane component and sustained residual hepcidin mRNA production. We speculate that the initial hypoxic injury or proteolytic damage to membrane components and the subsequent progressive loss of iron sensing and signaling machinery may account for the somewhat smaller hepcidin induction by holotransferrin in the in vitro hepatocyte culture system (2- to 4-fold induction after 18-24 hours treatment) when compared with in vivo models, in which iron ingestion caused 3- to 5-fold induction of urinary hepcidin within 12 hours in humans (Figure 1) and approximately 10-fold induction of hepcidin mRNA concentration within 24 hours in mice.13
A puzzling feature of the iron-sensing system in hepatocytes and in vivo is that it responds to physiologic (ie, micromolar) concentrations of holoTf even though the known (holo) transferrin receptors TfR1 and TfR2 have much lower affinity constants for holoTf (KD2 = 29 nM and 350 nM, respectively)34 and so should be fully saturated at physiologic concentrations. It is formally possible that another protein with lower affinity for holoTf functions as its homeostatic receptor. It should be considered, however, that the binding affinity of holoTf to TfR1 or TfR2 was determined using only overexpressed forms of TfR1 or TfR2 and not in complex with other hepatocyte proteins. Affinity for holotransferrin may be different in TfR-containing complexes either due to the change in affinity of the original holoTf binding sites on the TfRs or the appearance of new low-affinity binding sites in the complex. Indeed, the binding affinity of holoTf to TfR1 has been shown to be reduced upon TfR1 binding to HFE.35,36 Moreover, the stability of TfR2 protein was regulated in the physiologic range of holoTf concentration (micromolar) only in hepatic cell lines and not when TfR2 was overexpressed in nonhepatic cell lines.28,29 This indicates that liver-specific proteins, presumably components of the iron-sensing complex, may be required for the stabilization of TfR2 by holoTf and presumably alter their interaction. Considering the requirement of TfR2 in hepcidin production37,38 and the regulation of its stability by physiologic holotransferrin concentrations,28,29 we speculate that TfR2 associated with the hepatocyte iron-sensing complex will have lower (micromolar range) affinity for holotransferrin.
A recent study8 showed that hemojuvelin functions as a BMP coreceptor in regulating hepcidin expression but did not examine its role in hepcidin regulation by iron. Our work complements and extends the work of Babitt et al8 and establishes that both hemojuvelin and BMP2/4 are components of the iron-sensing and signaling pathway that regulates hepcidin synthesis.
In addition to BMP2 and 4, BMP9 is also a potent inducer of hepcidin mRNA expression.20 In adult animals, BMP9 is predominantly expressed in liver endothelial, Kupffer, and stellate cells.39 BMP9 was shown to bind HepG2 cells, liver endothelial cells, and Kupffer cells through a specific receptor.39,40 Moreover, hepcidin mRNA expression was strongly induced in a hepatocyte/endothelial cell coculture system,41 suggesting the possible interaction between the hepatocytes and the BMP9 produced by endothelial cells. During the isolation of hepatocytes, these cells are separated from nonparenchymal liver cells that produce BMP9, and the loss of BMP9 stimulation could contribute to the observed decline of hepcidin mRNA after hepatocyte isolation. Although BMP9 signaling increases the baseline hepcidin expression, BMP9 does not functionally interact with hemojuvelin and does not appear to participate in the iron regulation of hepcidin. Unlike BMP2/4, which bind to heterodimeric BMP receptor (BMPR) containing subunits BMPRIa or BMPRIb (also called activin-like receptor kinases ALK3 or ALK6) combined with BMPRII, BMP9 binds to other members of the TGF-β receptor family, including ALK1, ALK2, and coreceptor endoglin, and signals through these in combination with BMPR II or activin receptor ActRII.42 Because all of the BMP receptor systems use the same set of Smads, it is likely that the hemojuvelin/BMP2/4 and BMP9 signaling pathways converge at the level of the activated Smad1/5/8.
Thus, hepatocytes not only produce and secrete the iron-regulatory hormone hepcidin but also function as cellular sensors of plasma holotransferrin concentration, which reflects systemic iron balance. The iron-sensing and signaling pathway that is emerging from recent studies is unexpectedly complex and involves BMP2/4 and their receptors in combination with the GPI-linked coreceptor hemojuvelin and the genetically identified components transferrin receptor 2 and HFE. The in vitro model we described is readily applicable to hepatocytes from transgenic mice in which specific components of the proposed pathway are genetically altered and should help elucidate the roles and interactions of the multiple components of the iron-regulatory machinery.
Acknowledgments
We thank Dr Genhong Cheng for providing MyD88-deficient mice, Dr Ernest Beutler for sharing a hepatocyte purification protocol, and Dr Renata Stripecke from UCLA Vector Core Facility for lentiviral packaging. We are grateful to Yen Phung, Chun-Ling Jung, and Dharma Thapa for their technical assistance.
This work was supported by grants from the National Institutes of Health (NIH; KO1 DK07538, E.N.; RO1 DK065029 and R21 DK073226, T.G.) and the Will Rogers Fund (T.G.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.L. designed, performed, and analyzed the experiments and wrote the paper; E.V.V., J.B.G., and V.G. developed methods and performed experiments; E.N. designed and performed experiments and edited the paper; and T.G. designed experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tomas Ganz, Department of Medicine, CHS 37-055, David Geffen School of Medicine, Los Angeles, CA 90095-1690; e-mail: tganz@mednet.ucla.edu.
References
- 1.Ganz T, Nemeth E. Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta. 2006;1763:690–699. doi: 10.1016/j.bbamcr.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 3.Frazer DM, Wilkins SJ, Becker EM, et al. Hepcidin expression inversely correlates with the expression of duodenal iron transporters and iron absorption in rats. Gastroenterology. 2002;123:835–844. doi: 10.1053/gast.2002.35353. [DOI] [PubMed] [Google Scholar]
- 4.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin L, Goldberg YP, Ganz T. Competitive regulation of hepcidin mRNA by soluble and cell-associated hemojuvelin. Blood. 2005;106:2884–2889. doi: 10.1182/blood-2005-05-1845. [DOI] [PubMed] [Google Scholar]
- 7.Zhang AS, Anderson SA, Meyers KR, et al. Evidence that inhibition of hemojuvelin shedding in response to iron is mediated through neogenin. J Biol Chem. 2007;282:12547–12556. doi: 10.1074/jbc.M608788200. [DOI] [PubMed] [Google Scholar]
- 8.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 9.Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang RH, Li CL, Xu XL, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepicidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Shi YG, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemeth E, Valore EV, Territo M, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 15.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 16.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 17.Lee P, Peng HF, Gelbart T, Beutler E. The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta(2)-microglobulin-deficient hepatocytes. Proc Natl Acad Sci U S A. 2004;101:9263–9265. doi: 10.1073/pnas.0403108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee P, Peng HF, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A. 2005;102:1906–1910. doi: 10.1073/pnas.0409808102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Truksa J, Peng HF, Lee P, Beutler E. Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6. Proc Natl Acad Sci U S A. 2006;103:10289–10293. doi: 10.1073/pnas.0603124103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Groppe J, Greenwald J, Wiater E, et al. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 22.Sharov AA, Weiner L, Sharova TY, et al. Noggin overexpression inhibits eyelid opening by altering epidermal apoptosis and differentiation. EMBO J. 2003;22:2992–3003. doi: 10.1093/emboj/cdg291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.David L, Mallet C, Mazerbourg S, Feige JJ, Bailly S. Identification of BMP9 and BMP10 as functional activators of the orphan activin receptor-like kinase 1 (ALK1) endothelial cells. Blood. 2007;109:1953–1961. doi: 10.1182/blood-2006-07-034124. [DOI] [PubMed] [Google Scholar]
- 24.Bernstein SE. Hereditary hypotransferrinemia with hemosiderosis, a murine disorder resembling human atransferrinemia. J Lab Clin Med. 1987;110:690–705. [PubMed] [Google Scholar]
- 25.Beutler E, Gelbart T, Lee P, et al. Molecular characterization of a case of atransferrinemia. Blood. 2000;96:4071–4074. [PubMed] [Google Scholar]
- 26.Wilkins SJ, Frazer DM, Millard KN, McLaren GD, Anderson GJ. Iron metabolism in the hemoglobin-deficit mouse: correlation of diferric transferrin with hepcidin expression. Blood. 2006;107:1659–1664. doi: 10.1182/blood-2005-07-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawabata H, Fleming RE, Gui D, et al. Expression of hepcidin is down-regulated in TfR2 mutant mice manifesting a phenotype of hereditary hemochromatosis. Blood. 2005;105:376–381. doi: 10.1182/blood-2004-04-1416. [DOI] [PubMed] [Google Scholar]
- 28.Johnson MB, Enns CA. Diferric transferrin regulates transferrin receptor 2 protein stability. Blood. 2004;104:4287–4293. doi: 10.1182/blood-2004-06-2477. [DOI] [PubMed] [Google Scholar]
- 29.Robb A, Wessling-Resnick M. Regulation of transferrin receptor 2 protein levels by transferrin. Blood. 2004;104:4294–4299. doi: 10.1182/blood-2004-06-2481. [DOI] [PubMed] [Google Scholar]
- 30.Huebers H, Josephson B, Huebers E, Csiba E, Finch C. Uptake and release of iron from human transferrin. Proc Natl Acad Sci U S A. 1981;78:2572–2576. doi: 10.1073/pnas.78.4.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gehrke SG, Kulaksiz H, Herrmann T, et al. Expression of hepcidin in hereditary hemochromatosis: evidence for a regulation in response to the serum transferrin saturation and to non-transferrin-bound iron. Blood. 2003;102:371–376. doi: 10.1182/blood-2002-11-3610. [DOI] [PubMed] [Google Scholar]
- 32.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 33.Huebers HA, Josephson B, Huebers E, Csiba E, Finch CA. Occupancy of the iron-binding sites of human transferrin. Proc Natl Acad Sci U S A. 1984;81:4326–4330. doi: 10.1073/pnas.81.14.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West AP, Bennett MJ, Sellers VM, et al. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. 2000;275:38135–38138. doi: 10.1074/jbc.C000664200. [DOI] [PubMed] [Google Scholar]
- 35.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci U S A. 1998;95:1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebron JA, West AP, Bjorkman PJ. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. J Mol Biol. 1999;294:239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 37.Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105:1803–1806. doi: 10.1182/blood-2004-08-3042. [DOI] [PubMed] [Google Scholar]
- 38.Drake SF, Morgan EH, Herbison CE, et al. Iron absorption and hepatic iron uptake are increased in a transferrin receptor 2 (Y245X) mutant mouse model of hemochromatosis type 3. Am J Physiol Gastrointest Liver Physiol. 2007;292:G323–G328. doi: 10.1152/ajpgi.00278.2006. [DOI] [PubMed] [Google Scholar]
- 39.Miller AF, Harvey SAK, Thies RS, Olson MS. Bone morphogenetic protein-9: an autocrine/paracrine cytokine in the liver. J Biol Chem. 2000;275:17937–17945. doi: 10.1074/jbc.275.24.17937. [DOI] [PubMed] [Google Scholar]
- 40.Song JJ, Celeste AJ, Kong FM, et al. Bone morphogenetic protein-9 binds to liver cells and stimulates proliferation. Endocrinology. 1995;136:4293–4297. doi: 10.1210/endo.136.10.7664647. [DOI] [PubMed] [Google Scholar]
- 41.Takayama G, Taniguchi A, Okano T. Identification of differentially expressed genes in hepatocyte/endothelial cell co-culture system. Tissue Eng. 2007;13:159–166. doi: 10.1089/ten.2006.0143. [DOI] [PubMed] [Google Scholar]
- 42.Scharpfenecker M, van Dinther M, Liu Z, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–972. doi: 10.1242/jcs.002949. [DOI] [PubMed] [Google Scholar]