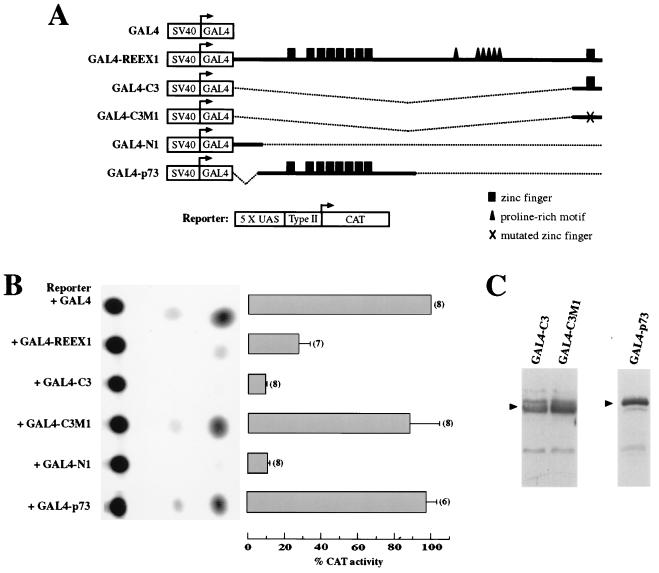
Figure 2.
Domains in the amino and carboxyl termini of REST are sufficient to mediate repression, and a point mutation in the zinc finger motif abrogates repressor activity. (A) Schematic representation of the family of GAL4-REST chimerical cDNAs expressed with a UAS type II-CAT reporter gene in transient transfections of PC12 cells. The reporter gene contains five copies of the UAS. The dotted lines indicate regions in REST that were deleted. Arrows indicate start sites for transcription. The carboxyl-terminal (C3), amino-terminal (N1), and mutated carboxyl-terminal (C3M1) fragments of REST are in-frame with the DNA-binding domain of the GAL4 protein. (B) (Left) Representative autoradiogram showing thin-layer chromatography (TLC) fractionation of acetylated forms of chloramphenicol. Each sample is from a dish of cells transfected transiently with the GAL4-REST chimerical cDNA and the UAS type II reporter gene. Note that the CAT assay with the GAL4-p73 construct was from a different TLC plate. (Right) Histogram showing compiled data from independent experiments. Standard errors of the mean and the numbers of experiments are indicated. (C) Western blot analysis of COS-1 cells transfected with the indicated GAL4-REST carboxyl-terminal (C3 and C3M1) and GAL4-p73 constructs. The amounts of the expressed proteins (arrowheads) show that the inability of the mutated carboxyl-terminal fragment C3M1 and p73 to repress is not due to instability of the expressed protein.