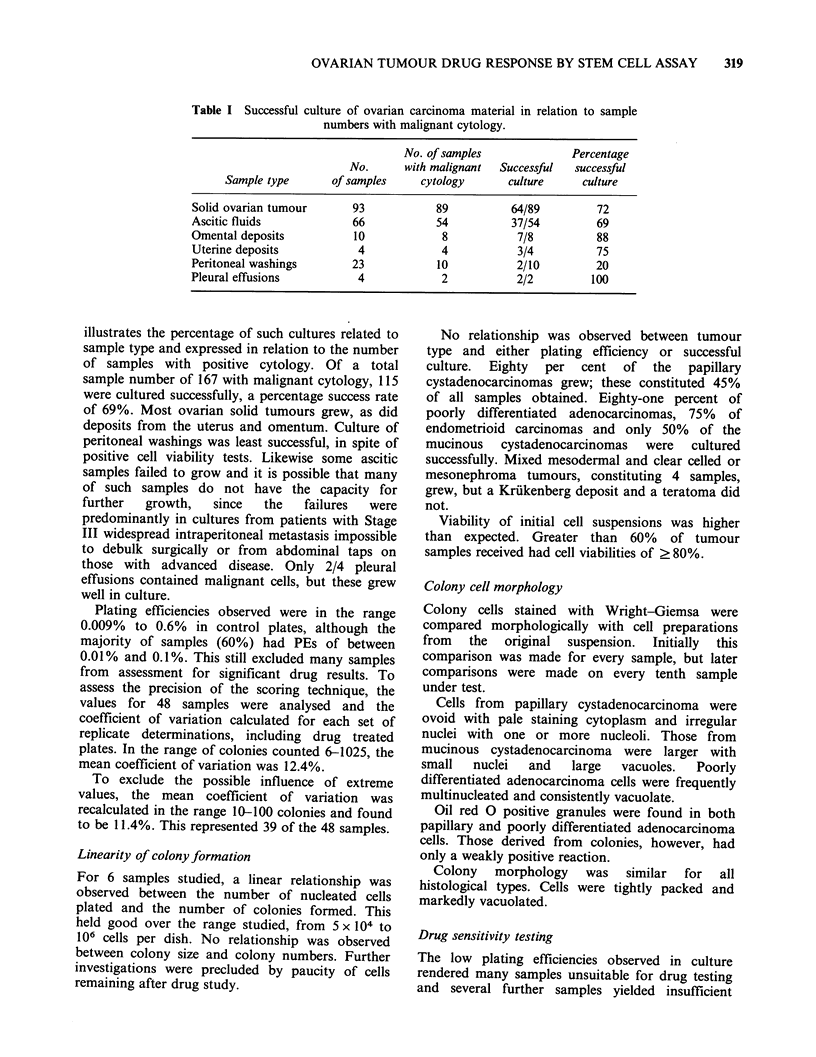
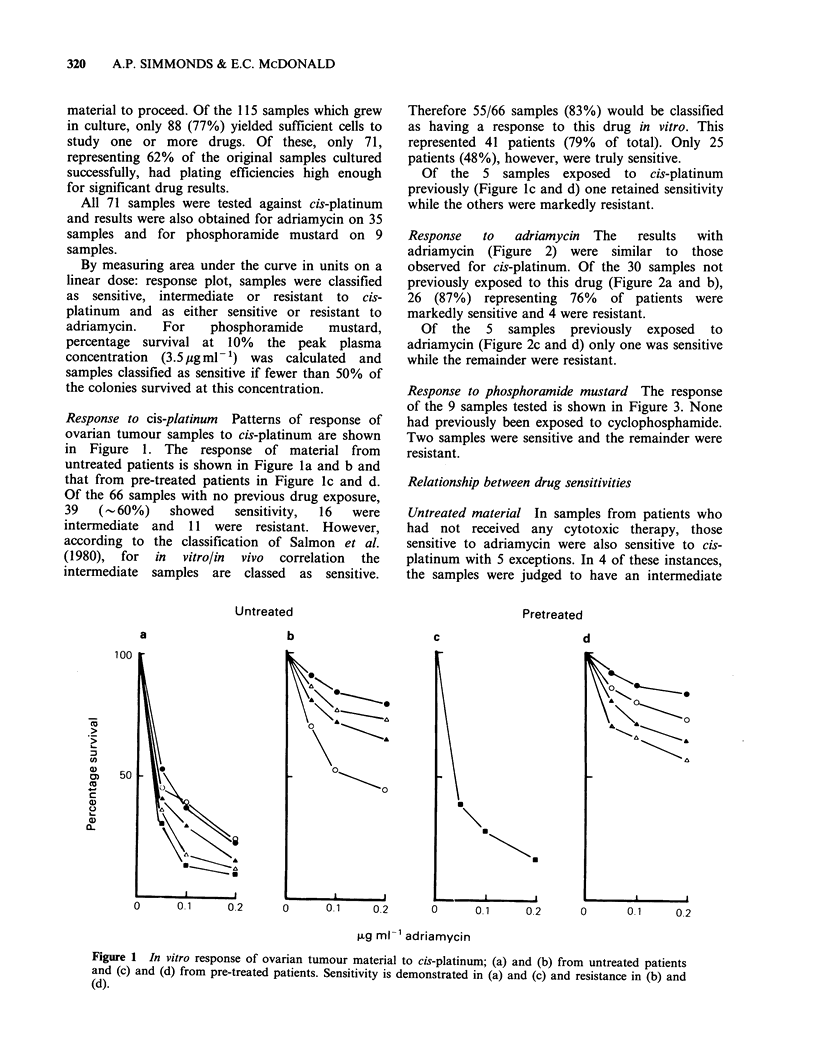
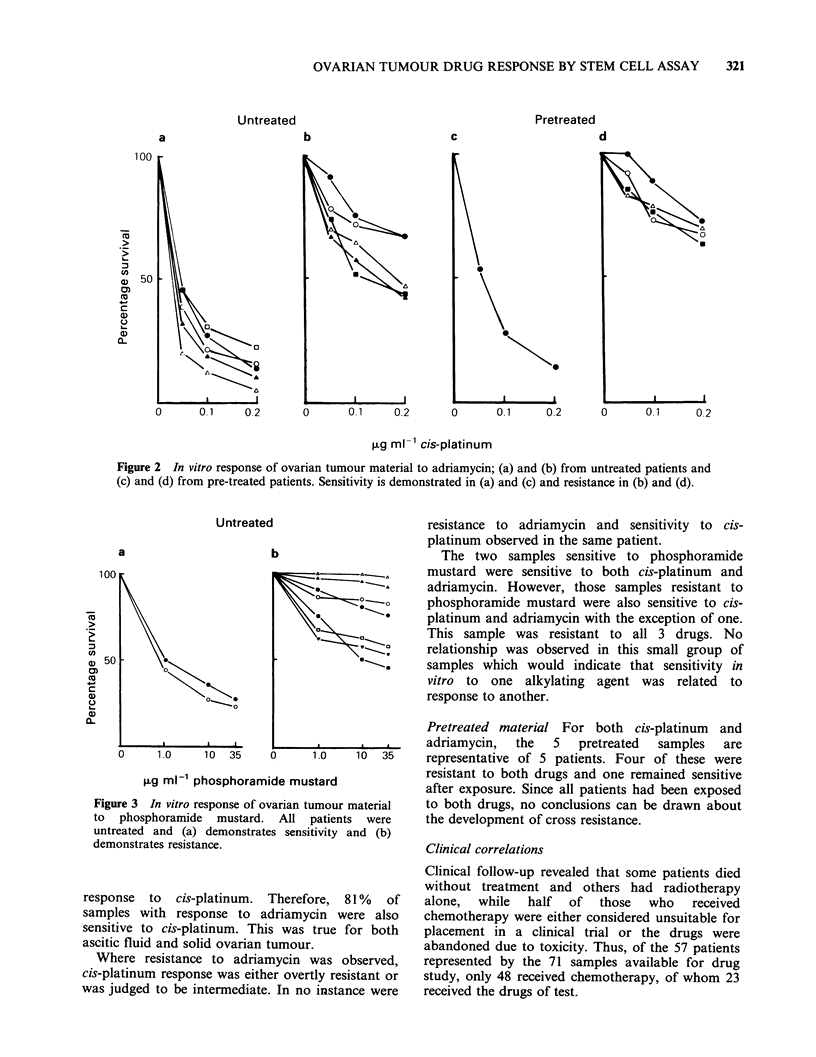
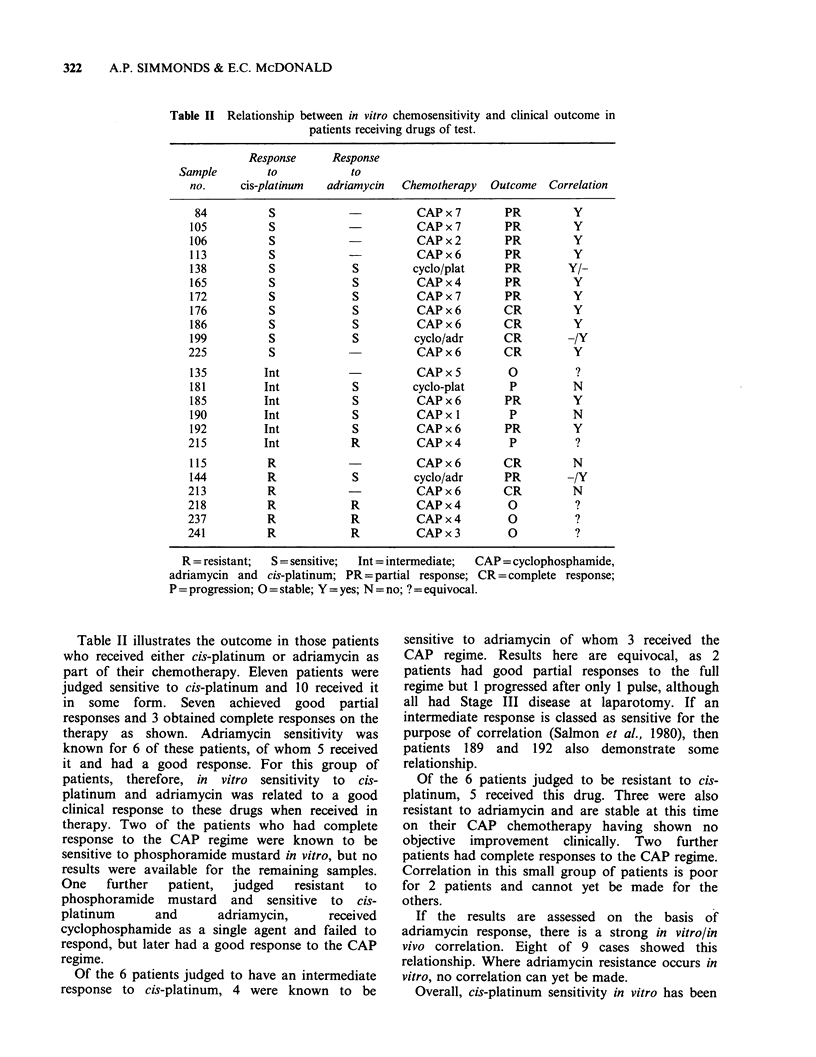
Abstract
Ovarian tumours were cultured by clonogenic assay and drug sensitivity profiles obtained for cis-platinum, adriamycin and phosphoramide mustard. Results were correlated with clinical outcome. Two hundred samples were received from 106 patients and 115/167 with malignant cytology (69%) were cultured successfully. Drug results were obtained on 71 samples and in untreated patients 60% of samples (48% of patients) were markedly sensitive to cis-platinum and 87% of samples (76% of patients) were sensitive to adriamycin. Eighty-one percent of cases sensitive to adriamycin were also sensitive to cis-platinum. Two of 7 samples were sensitive to phosphoramide mustard; the remainder were resistant. Eighty percent of samples from treated patients were resistant in vitro to drugs already received. Seventy-one samples from 57 patients were suitable for drug study. Forty-eight patients received chemotherapy, but only 23 received the drugs tested. Clinical correlations showed that in vitro sensitivity to cis-platinum and adriamycin was related to a good clinical response. No correlations were observed between cis-platinum and adriamycin resistance in vitro and clinical outcome. Unexpected relationships, however, were observed between cis-platinum resistance and failure to respond to other alkylating agents received singly. No such relationship has been demonstrated for adriamycin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertoncello I., Bradley T. R., Campbell J. J., Day A. J., McDonald I. A., McLeish G. R., Quinn M. A., Rome R., Hodgson G. S. Limitations of the clonal agar assay for the assessment of primary human ovarian tumour biopsies. Br J Cancer. 1982 Jun;45(6):803–811. doi: 10.1038/bjc.1982.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. J., Goldberg J. D., Holland J. F., Bruckner H. W., Deppe G., Gusberg S. B., Wallach R. C., Kabakow B., Rodin J. Improved therapy with cisplatin regimens for patients with ovarian carcinoma (FIGO Stages III and IV) as measured by surgical end-staging (second-look operation). Am J Obstet Gynecol. 1983 Apr 15;145(8):955–967. doi: 10.1016/0002-9378(83)90849-9. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E., Kim M. B., Trent J. M., Soehnlen B. J., Alberts D. S., Schmidt H. J. Direct cloning of human ovarian carcinoma cells in agar. Cancer Res. 1978 Oct;38(10):3438–3444. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Willson J. K., Grotzinger K. R., Young R. C. Cloning of human ovarian cancer cells in soft agar from malignant and peritoneal washings. Cancer Res. 1980 Aug;40(8 Pt 1):2743–2747. [PubMed] [Google Scholar]
- Ozols R. F., Willson J. K., Weltz M. D., Grotzinger K. R., Myers C. E., Young R. C. Inhibition of human ovarian cancer colony formation by adriamycin and its major metabolites. Cancer Res. 1980 Nov;40(11):4109–4112. [PubMed] [Google Scholar]
- Pavelic Z. P., Slocum H. K., Rustum Y. M., Creaven P. J., Nowak N. J., Karakousis C., Takita H., Mittelman A. Growth of cell colonies in soft agar from biopsies of different human solid tumors. Cancer Res. 1980 Nov;40(11):4151–4158. [PubMed] [Google Scholar]
- Powers J. F., Sladek N. E. Cytotoxic activity relative to 4-hydroxycyclophosphamide and phosphoramide mustard concentrations in the plasma of cyclophosphamide-treated rats. Cancer Res. 1983 Mar;43(3):1101–1106. [PubMed] [Google Scholar]
- Rupniak H. T., Hill B. T. The poor cloning ability in agar of human tumour cells from biopsies of primary tumours. Cell Biol Int Rep. 1980 May;4(5):479–486. doi: 10.1016/0309-1651(80)90035-1. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Alberts D. S., Meyskens F. L., Jr, Durie B. G., Jones S. E., Soehnlen B., Young L., Chen H. S., Moon T. E. Clinical correlations of in vitro drug sensitivity. Prog Clin Biol Res. 1980;48:223–245. [PubMed] [Google Scholar]
- Salmon S. E., Liu R. Direct "wet" staining of tumour or haematopoietic colonies in agar culture. Br J Cancer. 1979 Jun;39(6):779–781. doi: 10.1038/bjc.1979.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D. D., Casper J., Bradley E., Sandbach J., Jones D., Makuch R. Association between human tumor colony-forming assay results and response of an individual patient's tumor to chemotherapy. Am J Med. 1981 May;70(5):1027–1041. doi: 10.1016/0002-9343(81)90859-7. [DOI] [PubMed] [Google Scholar]
- Williams T. J., Lieber M. M., Podratz K. C., Malkasian G. D., Jr Soft agar colony formation assay for in vitro testing of sensitivity to chemotherapy of gynecologic malignancies. Am J Obstet Gynecol. 1983 Apr 15;145(8):940–947. doi: 10.1016/0002-9378(83)90845-1. [DOI] [PubMed] [Google Scholar]
- Wilson A. P., Neal F. E. In vitro sensitivity of human ovarian tumours to chemotherapeutic agents. Br J Cancer. 1981 Aug;44(2):189–200. doi: 10.1038/bjc.1981.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Palo G. M., de Lena M., Di Re F., Luciani L., Valagussa P., Bonadonna G. Melphalan versus adriamycin in the treatment of advanced carcinoma of the ovary. Surg Gynecol Obstet. 1975 Dec;141(6):899–902. [PubMed] [Google Scholar]


