Abstract
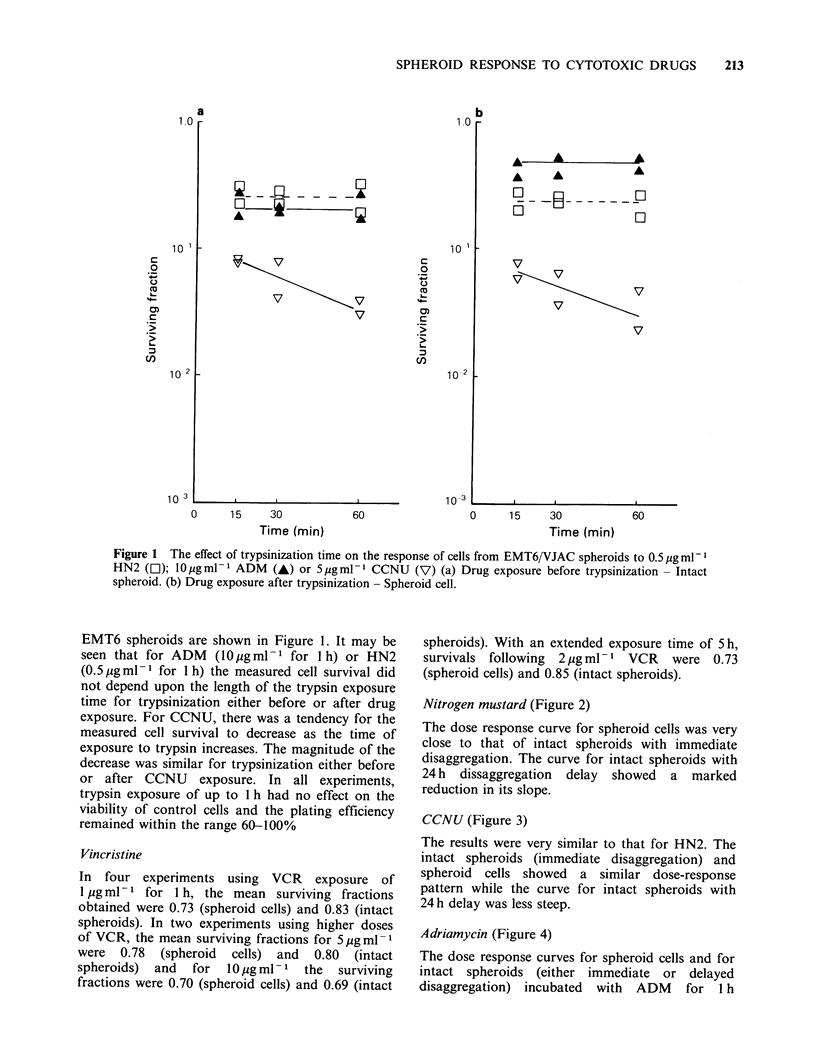
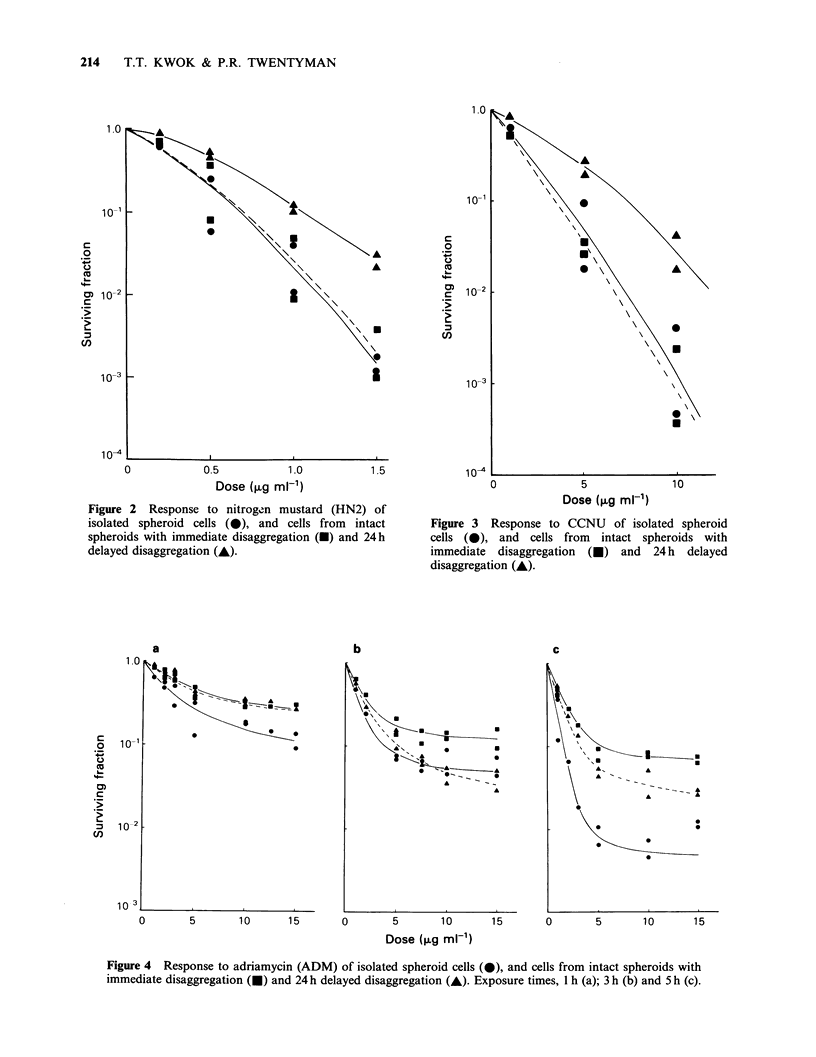
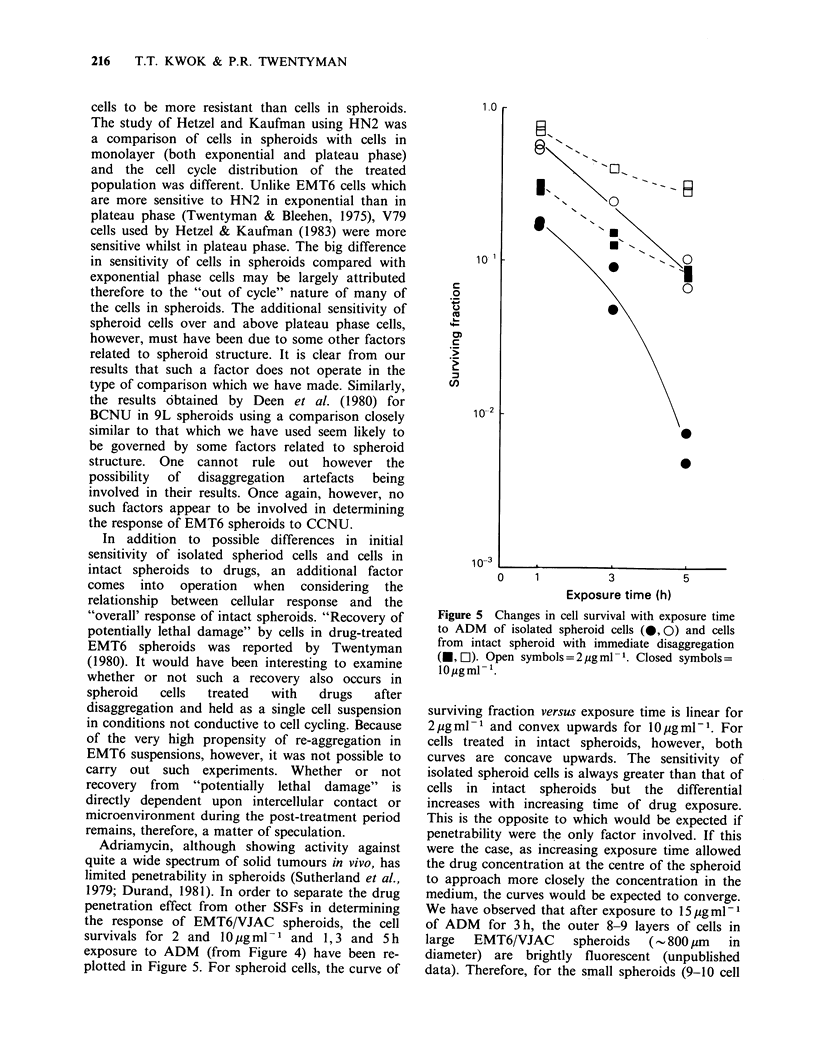
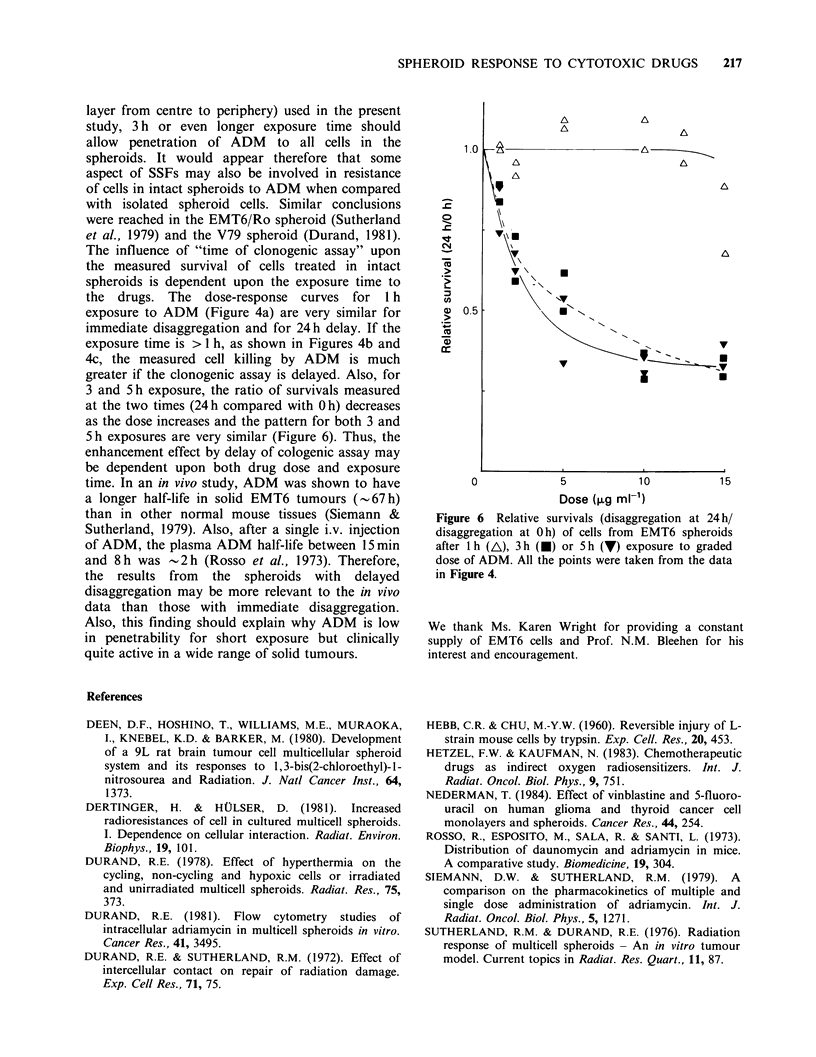
We have compared the response to a number of cytotoxic drugs of cells treated either within intact multicellular spheroids or as isolated cells following spheroid disaggregation. The cells used were of the EMT6/Ca/VJAC mouse tumour line and spheroids were treated or disaggregated at a mean diameter of 250 micron. The response of cell to nitrogen mustard (HN2) or CCNU was similar under the two exposure conditions and we conclude that factors related to spheroid structure (i.e. drug penetrability, intercellular contact effect and microenvironment within the spheroid) do not influence the initial response to these agents. Recovery of potentially lethal damage occurring over 24 h, however, greatly modifies the level of cell killing in intact spheroids. EMT6 cells were found to be extremely resistant to vincristine under all exposure conditions. For adriamycin (ADM), cells were always initially more sensitive when exposed to the drug in suspension rather than in intact spheroids. When ADM exposure was prolonged beyond 1 h, however, delaying spheroid disaggregation for 24 h led to increased cell kill and reduced differential between the two conditions of exposure. The data suggest that both drug penetration problems and other factors related to spheroid structure are involved in determining the response of cells in small spheroids to ADM.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deen D. F., Hoshino T., Williams M. E., Muraoka I., Knebel K. D., Barker M. Development of a 9L rat brain tumor cell multicellular spheroid system and its response to 1,3-bis(2-chloroethyl)-1-nitrosourea and radiation. J Natl Cancer Inst. 1980 Jun;64(6):1373–1382. doi: 10.1093/jnci/64.6.1373. [DOI] [PubMed] [Google Scholar]
- Dertinger H., Hülser D. Increased radioresistance of cells in cultured multicell spheroids. I. Dependence on cellular interaction. Radiat Environ Biophys. 1981;19(2):101–107. doi: 10.1007/BF01324226. [DOI] [PubMed] [Google Scholar]
- Durand R. E. Effects of hyperthermia on the cycling, noncycling, and hypoxic cells of irradiated and unirradiated multicell spheroids. Radiat Res. 1978 Aug;75(2):373–384. [PubMed] [Google Scholar]
- Durand R. E. Flow cytometry studies of intracellular adriamycin in multicell spheroids in vitro. Cancer Res. 1981 Sep;41(9 Pt 1):3495–3498. [PubMed] [Google Scholar]
- Durand R. E., Sutherland R. M. Effects of intercellular contact on repair of radiation damage. Exp Cell Res. 1972 Mar;71(1):75–80. doi: 10.1016/0014-4827(72)90265-0. [DOI] [PubMed] [Google Scholar]
- Hetzel F. W., Kaufman N. Chemotherapeutic drugs as indirect oxygen radiosensitizer. Int J Radiat Oncol Biol Phys. 1983 May;9(5):751–757. doi: 10.1016/0360-3016(83)90244-4. [DOI] [PubMed] [Google Scholar]
- Nederman T. Effects of vinblastine and 5-fluorouracil on human glioma and thyroid cancer cell monolayers and spheroids. Cancer Res. 1984 Jan;44(1):254–258. [PubMed] [Google Scholar]
- RAUT HEBB C., WANG CHU M. Y. Reversible injury of L-strain mouse cells by trypsin. Exp Cell Res. 1960 Aug;20:453–457. doi: 10.1016/0014-4827(60)90174-9. [DOI] [PubMed] [Google Scholar]
- Rosso R., Esposito M., Sala R., Santi L. Distribution of daunomycin and adriamycin in mice. A comparative study. Biomedicine. 1973 Jul 20;19(7):304–307. [PubMed] [Google Scholar]
- Siemann D. W., Sutherland R. M. A comparison of the pharmacokinetics of multiple and single dose administrations of adriamycin. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1271–1274. doi: 10.1016/0360-3016(79)90652-7. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Durand R. E. Radiation response of multicell spheroids--an in vitro tumour model. Curr Top Radiat Res Q. 1976 Jan;11(1):87–139. [PubMed] [Google Scholar]
- Sutherland R. M., Eddy H. A., Bareham B., Reich K., Vanantwerp D. Resistance to adriamycin in multicellular spheroids. Int J Radiat Oncol Biol Phys. 1979 Aug;5(8):1225–1230. doi: 10.1016/0360-3016(79)90643-6. [DOI] [PubMed] [Google Scholar]
- Triton T. R., Yee G. The anticancer agent adriamycin can be actively cytotoxic without entering cells. Science. 1982 Jul 16;217(4556):248–250. doi: 10.1126/science.7089561. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Bleehen N. M. Changes in sensitivity to cytotoxic agents occurring during the life history of monolayer cultures of a mouse tumour cell line. Br J Cancer. 1975 Apr;31(4):417–423. doi: 10.1038/bjc.1975.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twentyman P. R. Response to chemotherapy of EMT6 spheroids as measured by growth delay and cell survival. Br J Cancer. 1980 Aug;42(2):297–304. doi: 10.1038/bjc.1980.230. [DOI] [PMC free article] [PubMed] [Google Scholar]


