Abstract
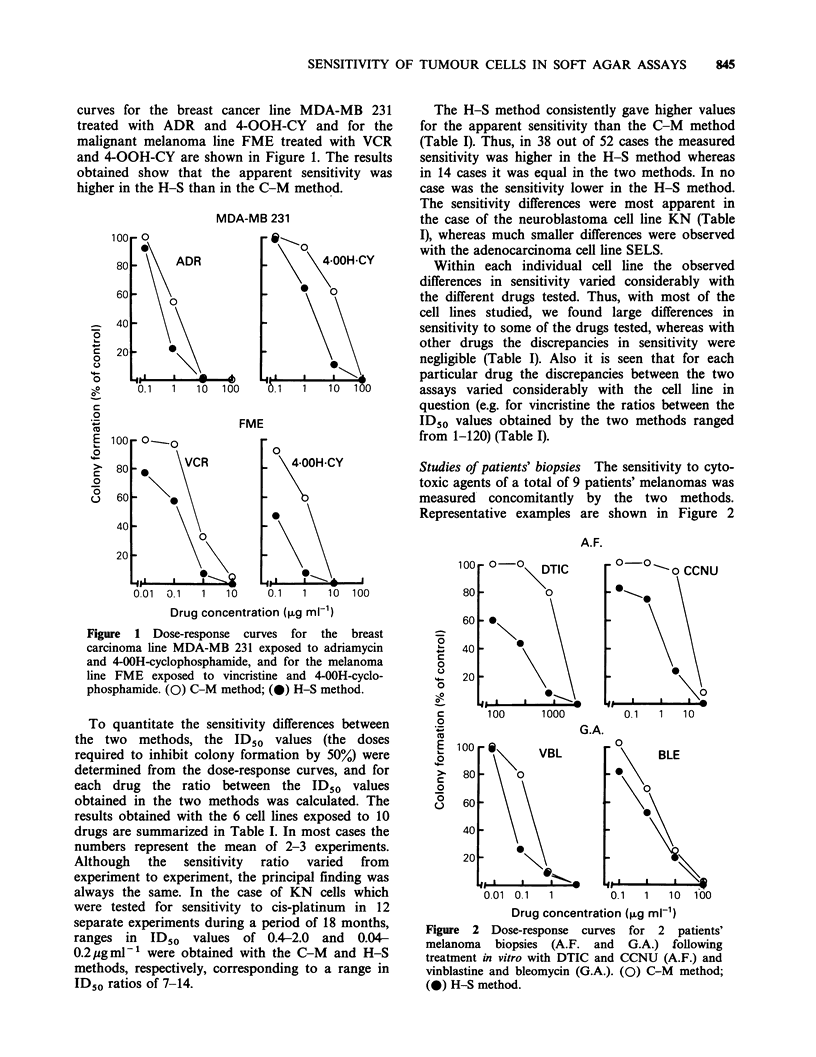
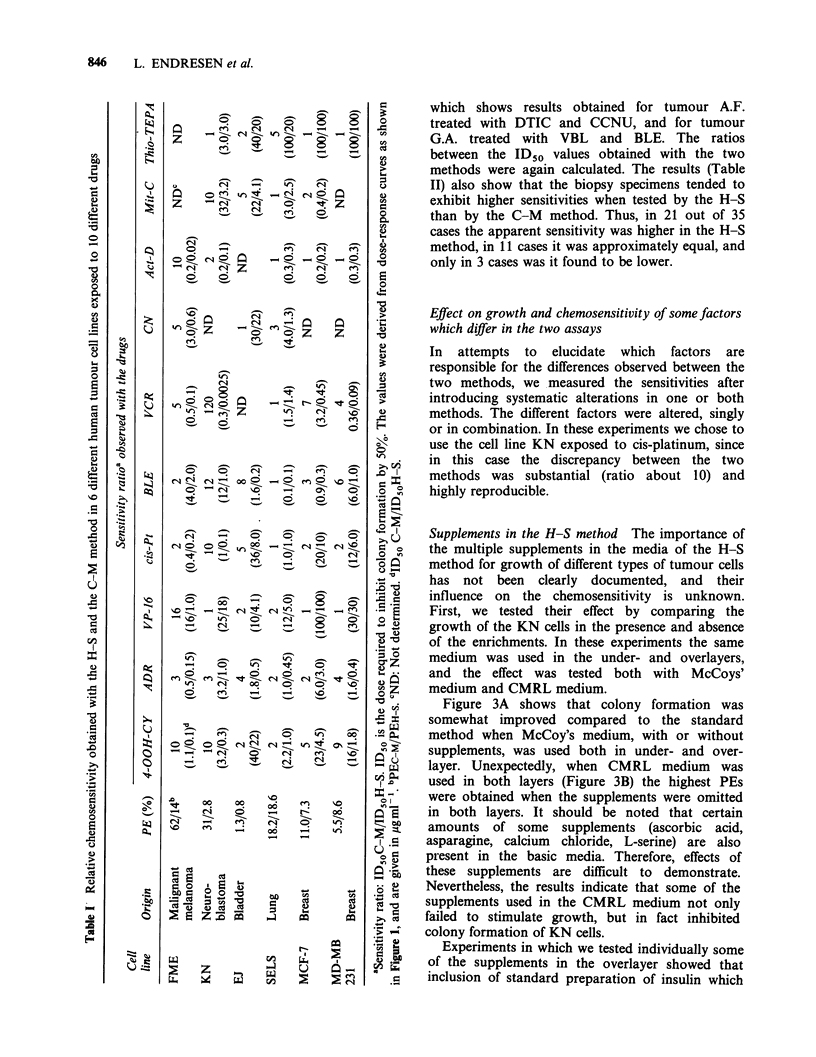
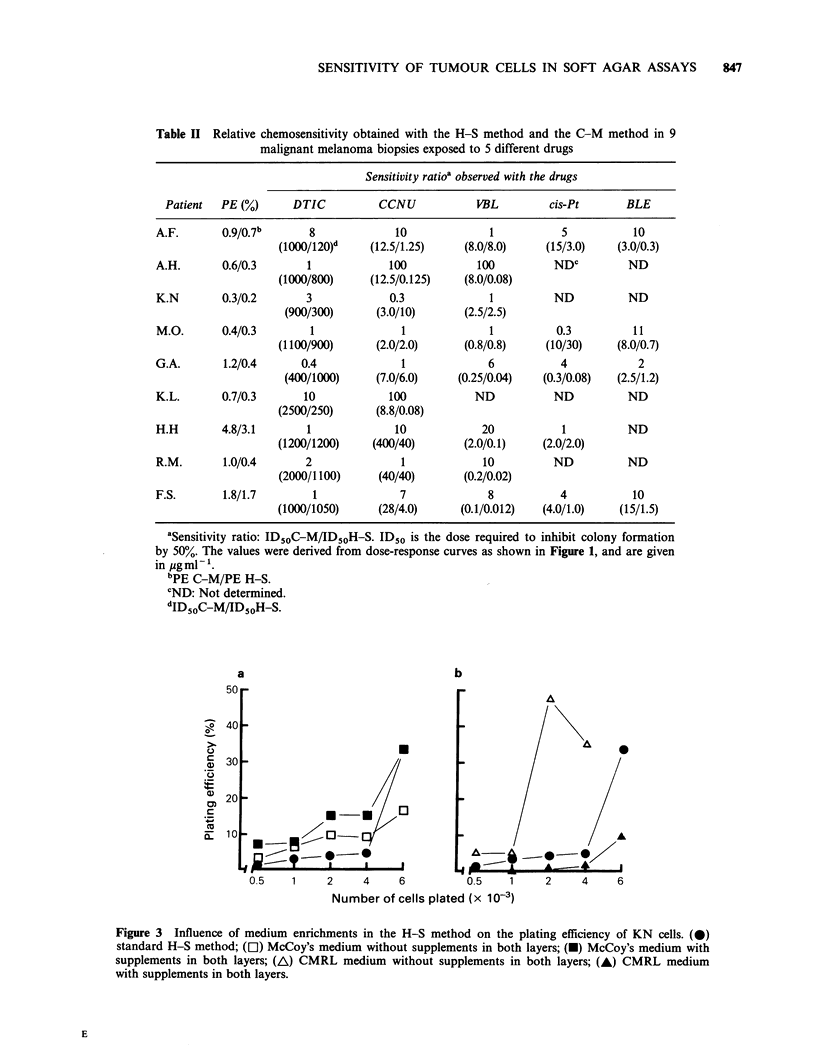
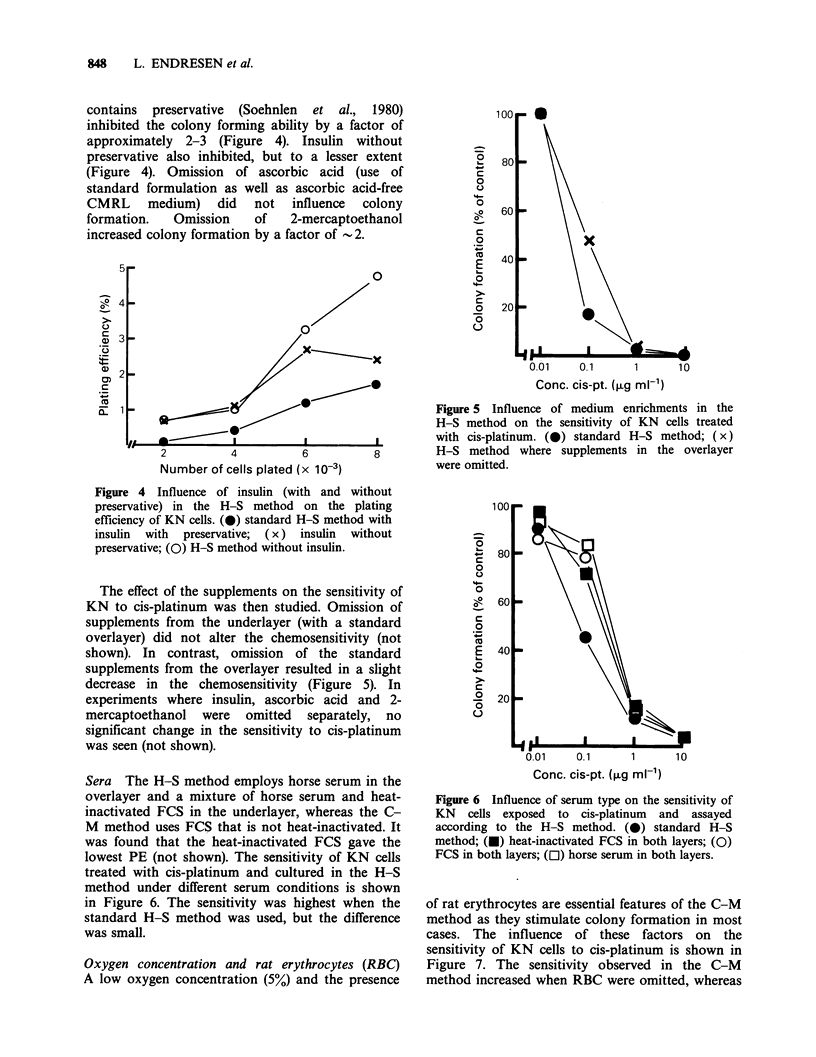
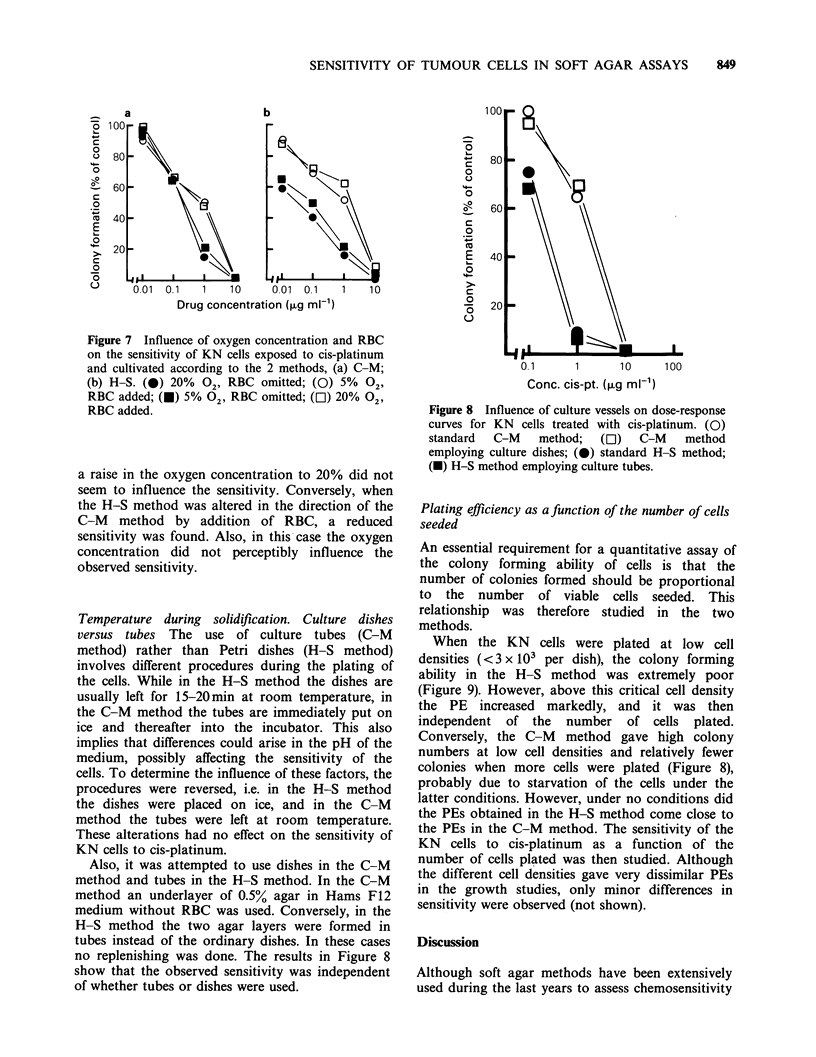
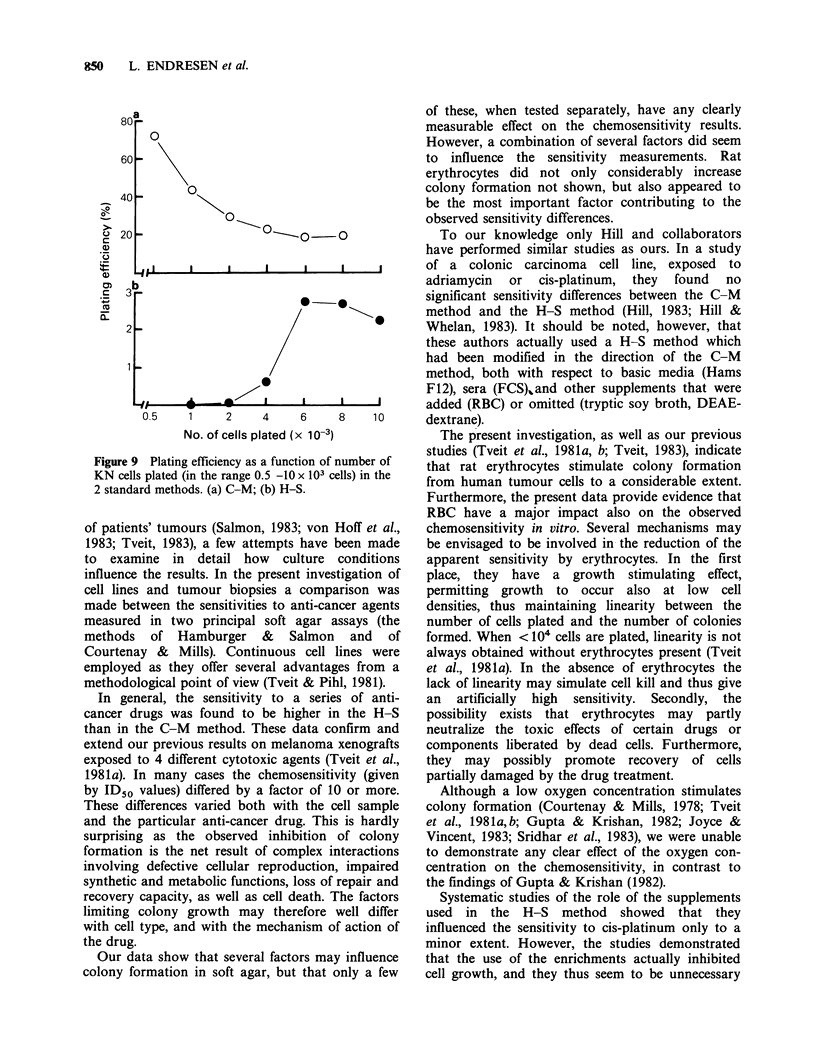
To investigate the influence of culture conditions on the in vitro responses of tumour cells to anticancer drugs, the sensitivities observed with the soft agar methods of Hamburger & Salmon (1977) (H-S) and of Courtenay & Mills (1978) (C-M) were compared. In all cases the ID50 values were determined from dose-response curves. Six human tumour cell lines exposed to 10 different agents, and 9 patients' melanomas exposed to 5 different agents, were examined. In the studies of cell lines the H-S method gave higher sensitivity values than the C-M method in 38 out of 52 cases, whereas in 14 cases the results were the same. In the patients' tumours the H-S method gave higher sensitivity in 21 of 35 cases, equal sensitivity in 11, and lower sensitivity in 3 cases. In many instances the ID50 values obtained with the two test systems differed by factors of 10 or more, both in the case of cell lines and tumour specimens. Systematic alterations in the culture conditions indicated that the presence or absence of rat erythrocytes is the most important factor responsible for the differences observed. Also, other factors, such as supplements (in the H-S method) and the use of different serum types, appeared to influence both colony growth and chemosensitivity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Courtenay V. D., Mills J. An in vitro colony assay for human tumours grown in immune-suppressed mice and treated in vivo with cytotoxic agents. Br J Cancer. 1978 Feb;37(2):261–268. doi: 10.1038/bjc.1978.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta V., Krishan A. Effect of oxygen concentration on the growth and drug sensitivity of human melanoma cells in soft-agar clonogenic assay. Cancer Res. 1982 Mar;42(3):1005–1007. [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hastings R. J., Franks L. M. Cellular heterogeneity in a tissue culture cell line derived from a human bladder carcinoma. Br J Cancer. 1983 Feb;47(2):233–244. doi: 10.1038/bjc.1983.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill B. T., Whelan R. D. Attempts to optimise colony-forming efficiencies using three different survival assays and a range of human tumour continuous cell lines. Cell Biol Int Rep. 1983 Aug;7(8):617–624. doi: 10.1016/0309-1651(83)90116-9. [DOI] [PubMed] [Google Scholar]
- Joyce R. M., Vincent P. C. Advantage of reduced oxygen tension in growth of human melanomas in semi-solid cultures: quantitative analysis. Br J Cancer. 1983 Sep;48(3):385–393. doi: 10.1038/bjc.1983.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh F. R., Evans T. L., Sikic B. I. Methodologic problems in clonogenic assays of spontaneous human tumors. Cancer Chemother Pharmacol. 1981;6(3):205–210. doi: 10.1007/BF00256972. [DOI] [PubMed] [Google Scholar]
- Salmon S. E. Human tumor colony assay and chemosensitivity testing. Cancer Treat Rep. 1984 Jan;68(1):117–125. [PubMed] [Google Scholar]
- Selby P., Buick R. N., Tannock I. A critical appraisal of the "human tumor stem-cell assay". N Engl J Med. 1983 Jan 20;308(3):129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- Soehnlen B., Young L., Liu R. Standard laboratory procedures for in vitro assay of human tumor stem cells. Prog Clin Biol Res. 1980;48:331–338. [PubMed] [Google Scholar]
- Sridhar K. S., Plasse T. F., Holland J. F., Shapiro M., Ohnuma T. Effects of physiological oxygen concentration on human tumor colony growth in soft agar. Cancer Res. 1983 Oct;43(10):4629–4631. [PubMed] [Google Scholar]
- Tveit K. M., Endresen L., Rugstad H. E., Fodstad O., Pihl A. Comparison of two soft-agar methods for assaying chemosensitivity of human tumours in vitro: malignant melanomas. Br J Cancer. 1981 Oct;44(4):539–544. doi: 10.1038/bjc.1981.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Johannessen J. V., Olsnes S. A human melanoma cell line established from xenograft in athymic mice. Br J Cancer. 1980 May;41(5):724–733. doi: 10.1038/bjc.1980.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Lotsberg J., Vaage S., Pihl A. Colony growth and chemosensitivity in vitro of human melanoma biopsies. Relationship to clinical parameters. Int J Cancer. 1982 May 15;29(5):533–538. doi: 10.1002/ijc.2910290508. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Olsnes S., Pihl A. In vitro sensitivity of human melanoma xenografts to cytotoxic drugs. Correlation with in vivo chemosensitivity. Int J Cancer. 1980 Dec 15;26(6):717–722. doi: 10.1002/ijc.2910260604. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Fodstad O., Pihl A. Cultivation of human melanomas in soft agar. Factors influencing plating efficiency and chemosensitivity. Int J Cancer. 1981 Sep 15;28(3):329–334. doi: 10.1002/ijc.2910280312. [DOI] [PubMed] [Google Scholar]
- Tveit K. M., Pihl A. Do cell lines in vitro reflect the properties of the tumours of origin? A study of lines derived from human melanoma xenografts. Br J Cancer. 1981 Dec;44(6):775–786. doi: 10.1038/bjc.1981.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff D. D., Clark G. M., Stogdill B. J., Sarosdy M. F., O'Brien M. T., Casper J. T., Mattox D. E., Page C. P., Cruz A. B., Sandbach J. F. Prospective clinical trial of a human tumor cloning system. Cancer Res. 1983 Apr;43(4):1926–1931. [PubMed] [Google Scholar]


