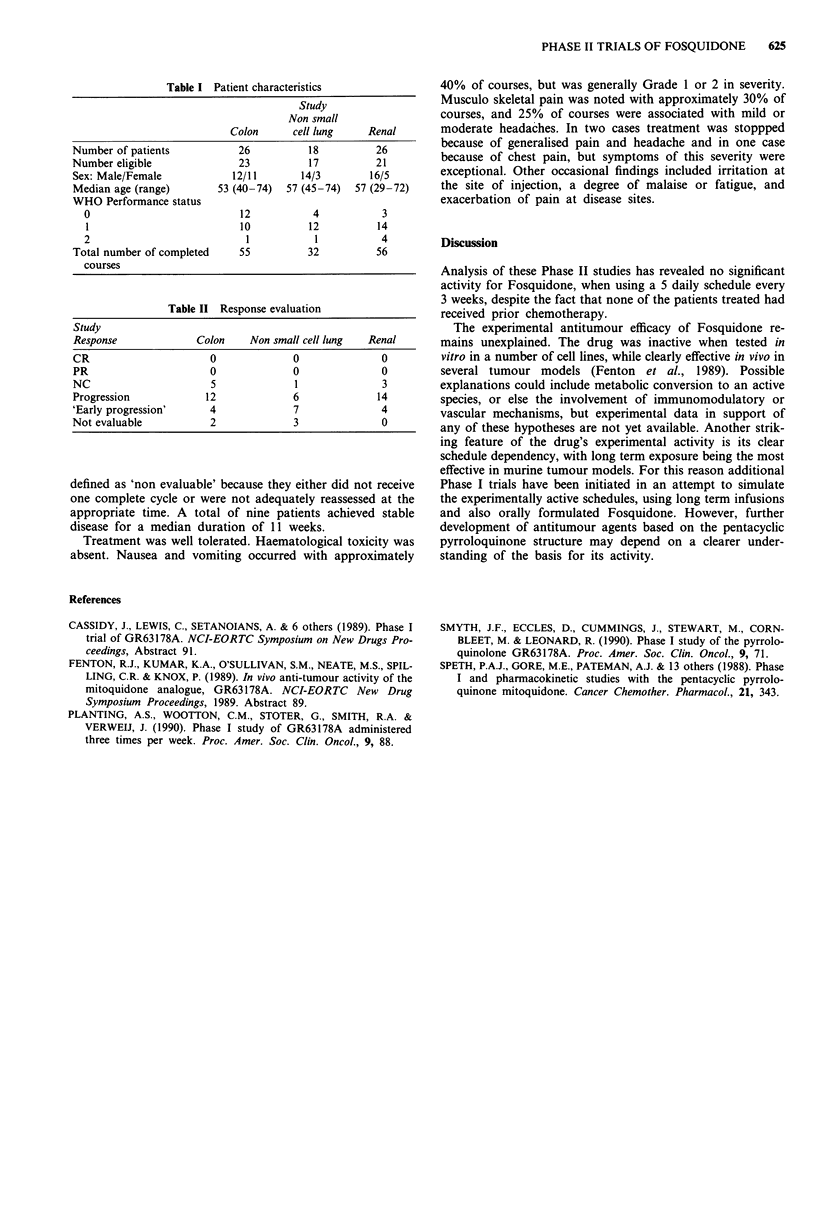
Abstract
A total of 61 eligible patients with metastatic cancer have been treated in a series of Phase II trials of the novel pentacyclic pyrroloquinone, fosquidone. Tumour types were colorectal (23), renal (21), and non small cell lung (17). No patient had received prior chemotherapy. The drug was given intravenously as a 20 min infusion at the dose of 120 mg-2 on days 1 to 5 every 3 weeks. Treatment was well tolerated; the only significant side effects being mild nausea and generalised musculo-skeletal pains. Response was assessed after two cycles of therapy. No patient achieved an objective partial response. A total of nine patients demonstrated stable disease for a median duration of 11 weeks. Using this schedule of administration, fosquidone has no significant antitumour activity in this group of tumours.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Speth P. A., Gore M. E., Pateman A. J., Newell D. R., Bishop J. A., Ellis W. J., Green J. A., Gumbrell L. A., Linssen P. C., Miller A. Phase I and pharmacokinetic studies with the pentacyclic pyrroloquinone mitoquidone. Cancer Chemother Pharmacol. 1988;21(4):343–346. doi: 10.1007/BF00264202. [DOI] [PubMed] [Google Scholar]


