Abstract
We have quantitated the levels of mRNAs in bone marrow samples from patients with multiple myeloma of the mdr1 gene (responsible for the Multidrug Resistance phenotype) and for two of the glutathione S-transferase gene, GST-2 and GST-3 (which can also inactivate a wide variety of cytotoxic drugs) and examined the relationship between the levels of expression of these genes and response to subsequent chemotherapy. From a total of 47 patients, 37 were treated with chemotherapy with 34 evaluable for response. Twenty-nine of the patients treated had not received any treatment prior to the marrow sampling while eight had previously received chemotherapy. Patients who failed to respond to initial chemotherapy had significantly higher levels of mdr1 than patients who responded (P = 0.01). In the total myeloma patient data set, mRNA levels for mdr1 and GST-2 were significantly correlated (Spearman rank correlation coefficient (r) = 0.54, P = 0.0004) as were expression levels of GST-2 with GST-3 (r = 0.43, P = 0.017). GST-3 and mdr1 levels were more weekly associated (r = 0.16, P = 0.4). These data would suggest a significant relationship between failure of chemotherapy in multiple myeloma patients and increases in expression of the mdr1 gene together with other genes whose products will generate additional mechanisms of resistance to chemotherapeutic agents.
Full text
PDF
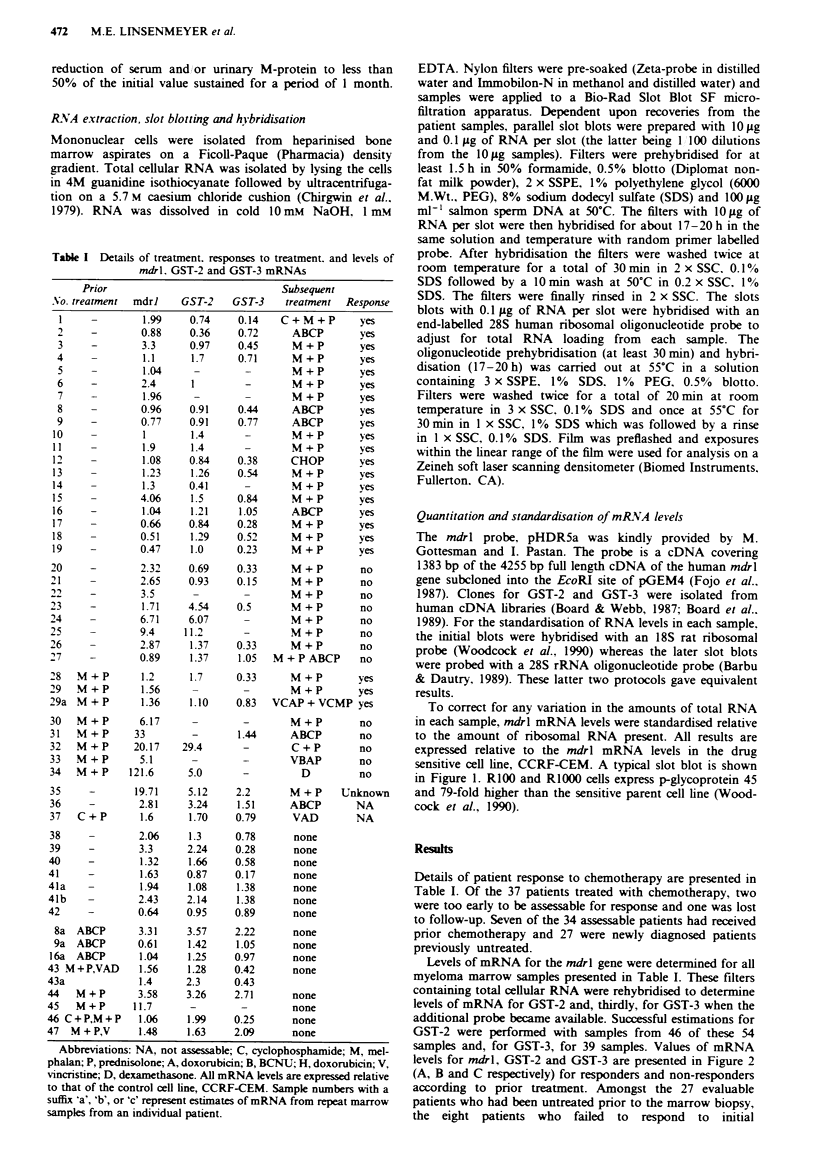
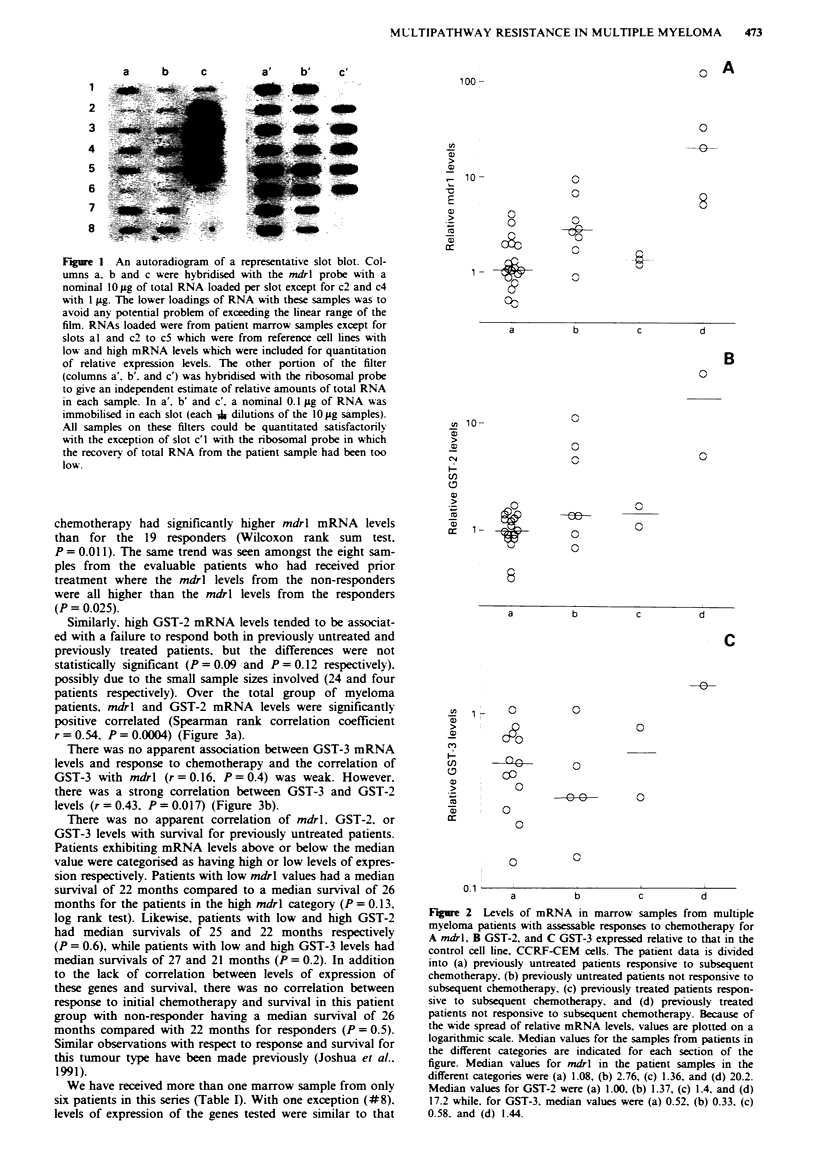
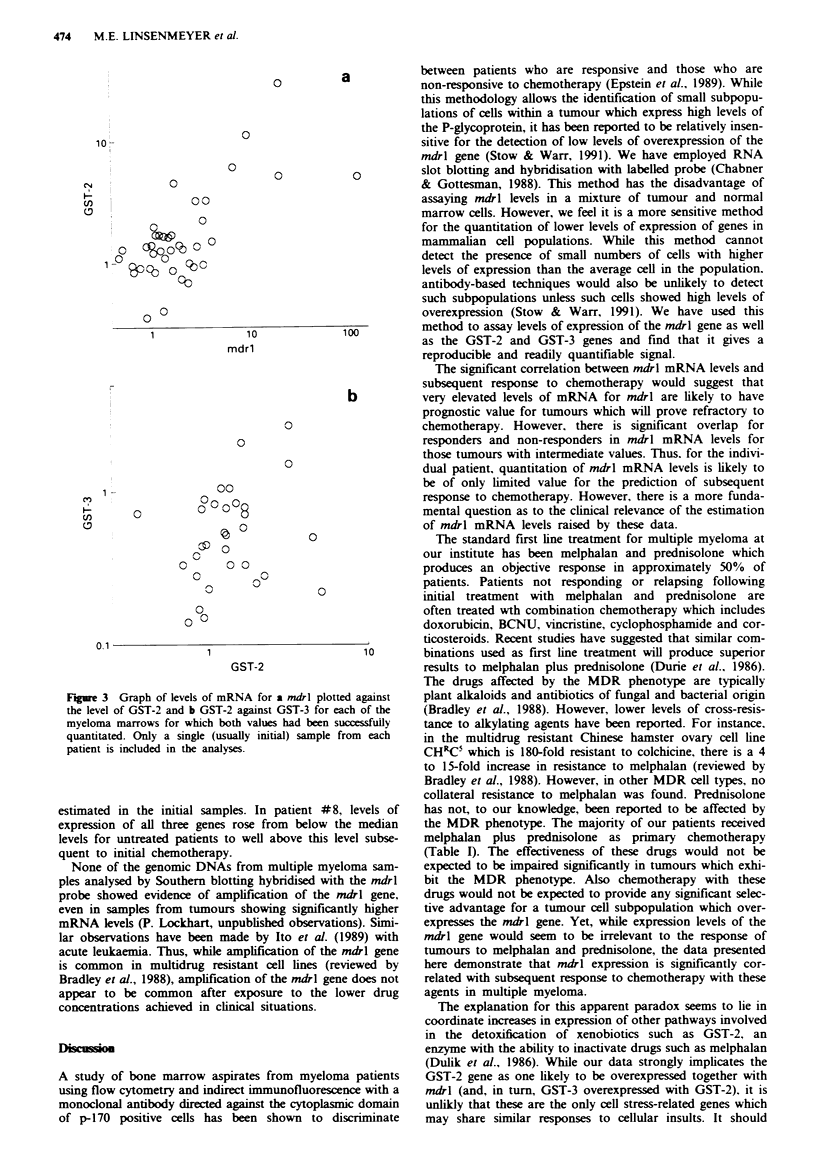

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbu V., Dautry F. Northern blot normalization with a 28S rRNA oligonucleotide probe. Nucleic Acids Res. 1989 Sep 12;17(17):7115–7115. doi: 10.1093/nar/17.17.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlogie B., Smith L., Alexanian R. Effective treatment of advanced multiple myeloma refractory to alkylating agents. N Engl J Med. 1984 May 24;310(21):1353–1356. doi: 10.1056/NEJM198405243102104. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C., Coggan M. Isolation of a cDNA clone and localization of the human glutathione S-transferase 3 genes to chromosome bands 11q13 and 12q13-14. Ann Hum Genet. 1989 Jul;53(Pt 3):205–213. doi: 10.1111/j.1469-1809.1989.tb01786.x. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Juranka P. F., Ling V. Mechanism of multidrug resistance. Biochim Biophys Acta. 1988 Aug 3;948(1):87–128. doi: 10.1016/0304-419x(88)90006-6. [DOI] [PubMed] [Google Scholar]
- Chabner B. A., Gottesman M. M. Meeting highlights: William Guy Forbeck Foundation think tank on "multidrug resistance in cancer chemotherapy". J Natl Cancer Inst. 1988 May 18;80(6):391–394. doi: 10.1093/jnci/80.6.391. [DOI] [PubMed] [Google Scholar]
- Chin K. V., Tanaka S., Darlington G., Pastan I., Gottesman M. M. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990 Jan 5;265(1):221–226. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalton W. S., Grogan T. M., Meltzer P. S., Scheper R. J., Durie B. G., Taylor C. W., Miller T. P., Salmon S. E. Drug-resistance in multiple myeloma and non-Hodgkin's lymphoma: detection of P-glycoprotein and potential circumvention by addition of verapamil to chemotherapy. J Clin Oncol. 1989 Apr;7(4):415–424. doi: 10.1200/JCO.1989.7.4.415. [DOI] [PubMed] [Google Scholar]
- Dulik D. M., Fenselau C., Hilton J. Characterization of melphalan-glutathione adducts whose formation is catalyzed by glutathione transferases. Biochem Pharmacol. 1986 Oct 1;35(19):3405–3409. doi: 10.1016/0006-2952(86)90444-2. [DOI] [PubMed] [Google Scholar]
- Durie B. G., Dixon D. O., Carter S., Stephens R., Rivkin S., Bonnet J., Salmon S. E., Dabich L., Files J. C., Costanzi J. J. Improved survival duration with combination chemotherapy induction for multiple myeloma: a Southwest Oncology Group Study. J Clin Oncol. 1986 Aug;4(8):1227–1237. doi: 10.1200/JCO.1986.4.8.1227. [DOI] [PubMed] [Google Scholar]
- Epstein J., Xiao H. Q., Oba B. K. P-glycoprotein expression in plasma-cell myeloma is associated with resistance to VAD. Blood. 1989 Aug 15;74(3):913–917. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fojo A., Akiyama S., Gottesman M. M., Pastan I. Reduced drug accumulation in multiply drug-resistant human KB carcinoma cell lines. Cancer Res. 1985 Jul;45(7):3002–3007. [PubMed] [Google Scholar]
- Gros P., Croop J., Roninson I., Varshavsky A., Housman D. E. Isolation and characterization of DNA sequences amplified in multidrug-resistant hamster cells. Proc Natl Acad Sci U S A. 1986 Jan;83(2):337–341. doi: 10.1073/pnas.83.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tanimoto M., Kumazawa T., Okumura M., Morishima Y., Ohno R., Saito H. Increased P-glycoprotein expression and multidrug-resistant gene (mdr1) amplification are infrequently found in fresh acute leukemia cells. Sequential analysis of 15 cases at initial presentation and relapsed stage. Cancer. 1989 Apr 15;63(8):1534–1538. doi: 10.1002/1097-0142(19890415)63:8<1534::aid-cncr2820630813>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Kartner N., Riordan J. R., Ling V. Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines. Science. 1983 Sep 23;221(4617):1285–1288. doi: 10.1126/science.6137059. [DOI] [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988 Nov 25;263(33):17205–17208. [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M., Miyachi H., Sowers L. C., Rossi J. Molecular basis of cisplatin resistance in human carcinomas: model systems and patients. Anticancer Res. 1989 Sep-Oct;9(5):1301–1312. [PubMed] [Google Scholar]
- Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., Willingham M. C. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7735–7738. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson S. S., Huber B. E., Sorrell S., Fojo A., Pastan I., Gottesman M. M. Expression of the multidrug-resistant gene in hepatocarcinogenesis and regenerating rat liver. Science. 1987 May 29;236(4805):1120–1122. doi: 10.1126/science.3576227. [DOI] [PubMed] [Google Scholar]
- Woodcock D. M., Jefferson S., Linsenmeyer M. E., Crowther P. J., Chojnowski G. M., Williams B., Bertoncello I. Reversal of the multidrug resistance phenotype with cremophor EL, a common vehicle for water-insoluble vitamins and drugs. Cancer Res. 1990 Jul 15;50(14):4199–4203. [PubMed] [Google Scholar]