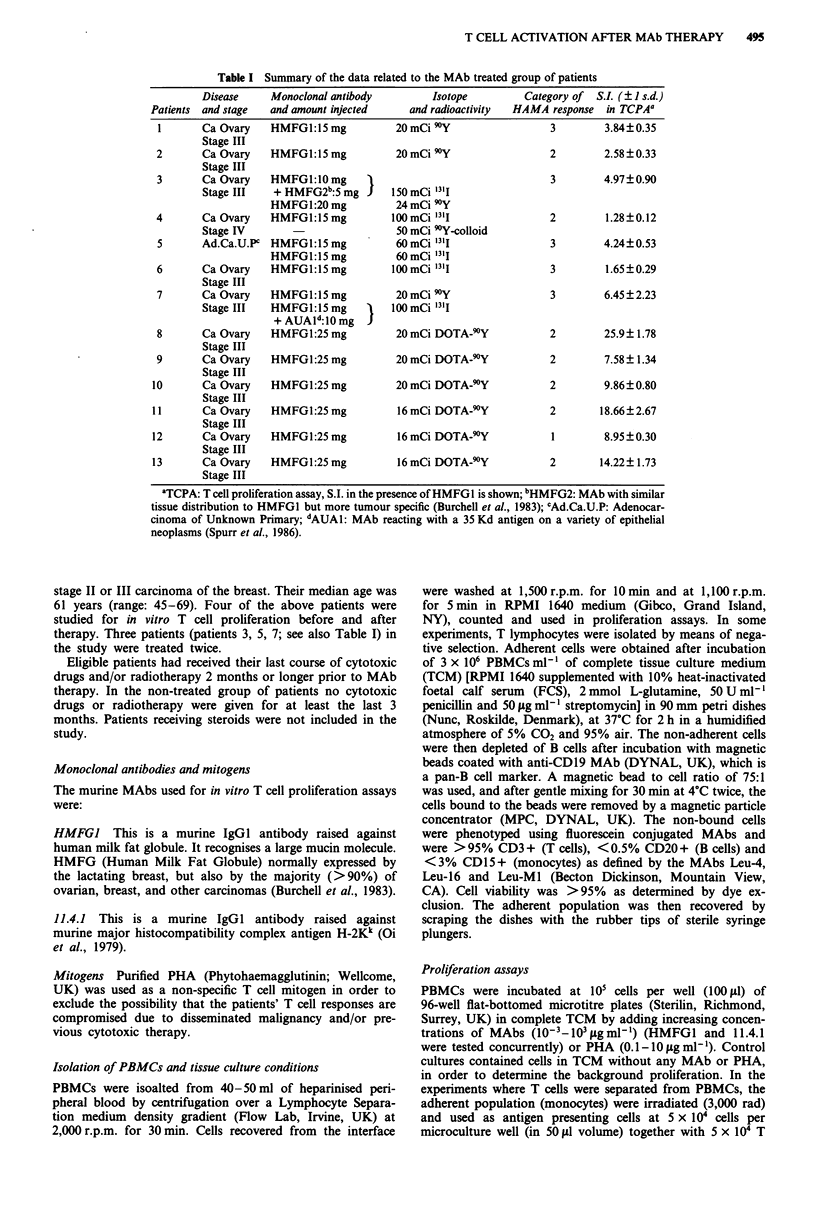
Abstract
Peripheral blood mononuclear cells (PBMCs) were obtained from patients receiving radioactive murine monoclonal antibody (MAb) therapy for malignant epithelial tumours, as well as normal controls, and were tested for the ability of T cells to proliferate in vitro in the presence of the MAb administered for therapy (HMFG1), and another isotypically matched antibody of irrelevant specificity (11.4.1). We studied 13 patients who had one (ten patients) or two (three patients) courses of MAB treatment, 11 age matched patients with the same histologic types of tumours, that had not received MAbs, and four normal controls. There was a consistent dose dependent in vitro T cell proliferation in 11 of the 13 patients after MAb therapy. This was not observed in the pre-therapy group of patients or normal controls, where the T cell proliferative responses remained baseline. The mean stimulation index (S.I.) in the post-therapy group was significantly higher than that of the pre-therapy patients and that of normal controls. When the in vitro T cell proliferative responses of these patients were measured in the presence of HMGF1 MAb (IgG1) and an isotypically identical, but idiotypically unrelated 11.4.1 MAb (IgG1), there was no statistically significant difference in the mean S.I. For HMFG1 vs 11.4.1 for the whole group of treated patients. When patients were separated into those who received one and those who received two MAb treatments, a significant increase in the mean S.I. was observed in the presence of HMFG1, in the group of patients receiving two treatment courses, suggesting the generation of T cells with specificity for the idiotypic component of the administered murine immunoglobulin. In order to further characterise these in vitro cellular responses we incubated PBMCs with and without an optimal concentration of the MAb (100-300 micrograms ml-1), as defined by the proliferation assay, and compared the differences in cell subpopulations. A significant increase in the percentage of cells expressing interleukin-2 receptors (IL-2R) was observed after MAb stimulation. The percentage of CD4+ lymphocytes and the CD4/CD8 ratio increased in all the cases studied, after MAb stimulation, where the percentages of B cells and NK cells remained relatively constant at less than 2-3% of the total population. We therefore conclude that murine MAbs administered to patients with cancer can lead to the generation of T cells which can recognise these MAbs as antigens when presented appropriately in vitro. The main proliferating population appears to be T helper CD4+ lymphocytes which following stimulation can release interleukin-2 leading to the expression of high levels of IL-2R.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burchell J., Durbin H., Taylor-Papadimitriou J. Complexity of expression of antigenic determinants, recognized by monoclonal antibodies HMFG-1 and HMFG-2, in normal and malignant human mammary epithelial cells. J Immunol. 1983 Jul;131(1):508–513. [PubMed] [Google Scholar]
- Chatenoud L., Baudrihaye M. F., Chkoff N., Kreis H., Goldstein G., Bach J. F. Restriction of the human in vivo immune response against the mouse monoclonal antibody OKT3. J Immunol. 1986 Aug 1;137(3):830–838. [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Moore R., Larche M., Pectasides D., Dhokia B., Ritter M. A. Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res. 1986 Dec;46(12 Pt 1):6489–6493. [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Sivolapenko G. B., Larche M., Barkans J. R., Ritter M. A. Development of anti-idiotypic antibodies against tumour antigens and autoantigens in ovarian cancer patients treated intraperitoneally with mouse monoclonal antibodies. Lancet. 1988 Oct 15;2(8616):894–897. doi: 10.1016/s0140-6736(88)92482-8. [DOI] [PubMed] [Google Scholar]
- Courtenay-Luck N. S., Epenetos A. A., Winearls C. G., Ritter M. A. Preexisting human anti-murine immunoglobulin reactivity due to polyclonal rheumatoid factors. Cancer Res. 1987 Aug 15;47(16):4520–4525. [PubMed] [Google Scholar]
- Epenetos A. A., Britton K. E., Mather S., Shepherd J., Granowska M., Taylor-Papadimitriou J., Nimmon C. C., Durbin H., Hawkins L. R., Malpas J. S. Targeting of iodine-123-labelled tumour-associated monoclonal antibodies to ovarian, breast, and gastrointestinal tumours. Lancet. 1982 Nov 6;2(8306):999–1005. doi: 10.1016/s0140-6736(82)90046-0. [DOI] [PubMed] [Google Scholar]
- Epenetos A. A., Munro A. J., Stewart S., Rampling R., Lambert H. E., McKenzie C. G., Soutter P., Rahemtulla A., Hooker G., Sivolapenko G. B. Antibody-guided irradiation of advanced ovarian cancer with intraperitoneally administered radiolabeled monoclonal antibodies. J Clin Oncol. 1987 Dec;5(12):1890–1899. doi: 10.1200/JCO.1987.5.12.1890. [DOI] [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Koprowski H., Herlyn D., Lubeck M., DeFreitas E., Sears H. F. Human anti-idiotype antibodies in cancer patients: Is the modulation of the immune response beneficial for the patient? Proc Natl Acad Sci U S A. 1984 Jan;81(1):216–219. doi: 10.1073/pnas.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A., Abrignani S., Scheidegger D., Obrist R., Dörken B., Moldenhauer G. Antibodies as antigens. The use of mouse monoclonal antibodies to focus human T cells against selected targets. J Exp Med. 1988 Feb 1;167(2):345–352. doi: 10.1084/jem.167.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Merkenschlager M., Terry L., Edwards R., Beverley P. C. Limiting dilution analysis of proliferative responses in human lymphocyte populations defined by the monoclonal antibody UCHL1: implications for differential CD45 expression in T cell memory formation. Eur J Immunol. 1988 Nov;18(11):1653–1661. doi: 10.1002/eji.1830181102. [DOI] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Oldham R. K., Foon K. A., Morgan A. C., Woodhouse C. S., Schroff R. W., Abrams P. G., Fer M., Schoenberger C. S., Farrell M., Kimball E. Monoclonal antibody therapy of malignant melanoma: in vivo localization in cutaneous metastasis after intravenous administration. J Clin Oncol. 1984 Nov;2(11):1235–1244. doi: 10.1200/JCO.1984.2.11.1235. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C., Rusk C. M. Low and high affinity cellular receptors for interleukin 2. Implications for the level of Tac antigen. J Exp Med. 1984 Oct 1;160(4):1126–1146. doi: 10.1084/jem.160.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y., Chen J. J., Shi L. F., Okuda Y., Köhler H. Idiotype-specific T helper clones recognize a variable H chain determinant. J Immunol. 1990 Mar 1;144(5):1625–1628. [PubMed] [Google Scholar]
- Saeki Y., Chen J. J., Shi L. F., Raychaudhuri S., Köhler H. Characterization of "regulatory" idiotope-specific T cell clones to a monoclonal anti-idiotypic antibody mimicking a tumor-associated antigen (TAA). J Immunol. 1989 Feb 1;142(3):1046–1052. [PubMed] [Google Scholar]
- Saltini C., Winestock K., Kirby M., Pinkston P., Crystal R. G. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N Engl J Med. 1989 Apr 27;320(17):1103–1109. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Schroff R. W., Foon K. A., Beatty S. M., Oldham R. K., Morgan A. C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985 Feb;45(2):879–885. [PubMed] [Google Scholar]
- Siccardi A. G., Buraggi G. L., Callegaro L., Colella A. C., De Filippi P. G., Galli G., Mariani G., Masi R., Palumbo R., Riva P. Immunoscintigraphy of adenocarcinomas by means of radiolabeled F(ab')2 fragments of an anti-carcinoembryonic antigen monoclonal antibody: a multicenter study. Cancer Res. 1989 Jun 1;49(11):3095–3103. [PubMed] [Google Scholar]
- Smith S. H., Brown M. H., Rowe D., Callard R. E., Beverley P. C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL1. Immunology. 1986 May;58(1):63–70. [PMC free article] [PubMed] [Google Scholar]
- Spurr N. K., Durbin H., Sheer D., Parkar M., Bobrow L., Bodmer W. F. Characterization and chromosomal assignment of a human cell surface antigen defined by the monoclonal antibody AUAI. Int J Cancer. 1986 Nov 15;38(5):631–636. doi: 10.1002/ijc.2910380503. [DOI] [PubMed] [Google Scholar]
- Stewart J. S., Hird V., Snook D., Dhokia B., Sivolapenko G., Hooker G., Papadimitriou J. T., Rowlinson G., Sullivan M., Lambert H. E. Intraperitoneal yttrium-90-labeled monoclonal antibody in ovarian cancer. J Clin Oncol. 1990 Dec;8(12):1941–1950. doi: 10.1200/JCO.1990.8.12.1941. [DOI] [PubMed] [Google Scholar]
- Traub U. C., DeJager R. L., Primus F. J., Losman M., Goldenberg D. M. Antiidiotype antibodies in cancer patients receiving monoclonal antibody to carcinoembryonic antigen. Cancer Res. 1988 Jul 15;48(14):4002–4006. [PubMed] [Google Scholar]


