Abstract
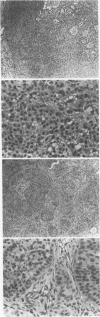
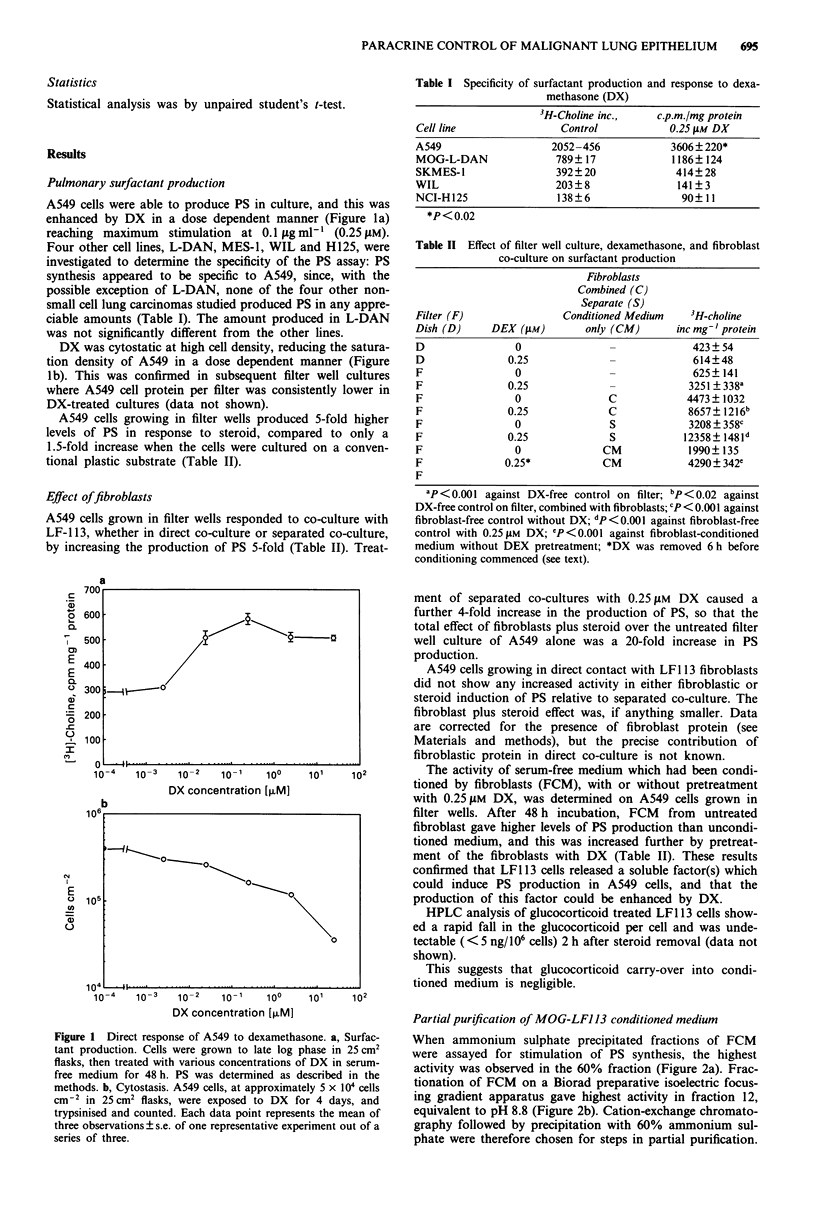
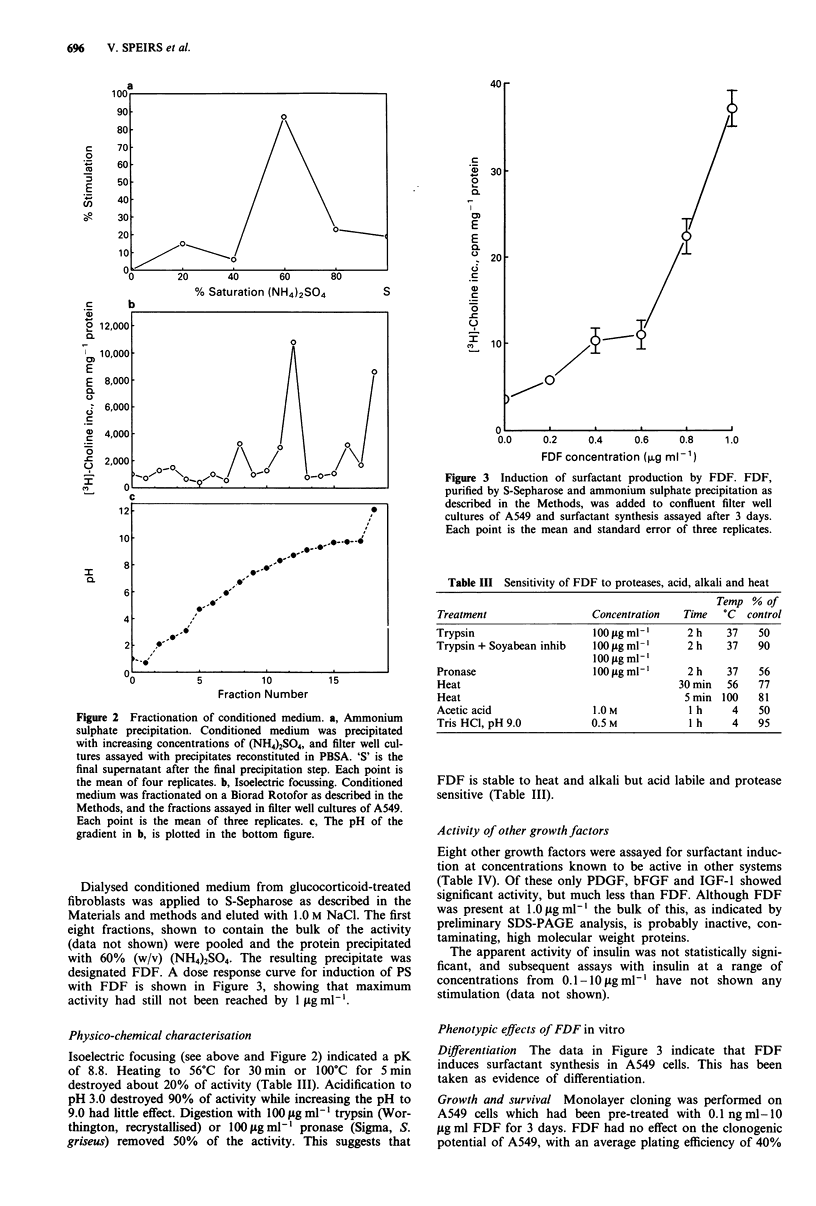
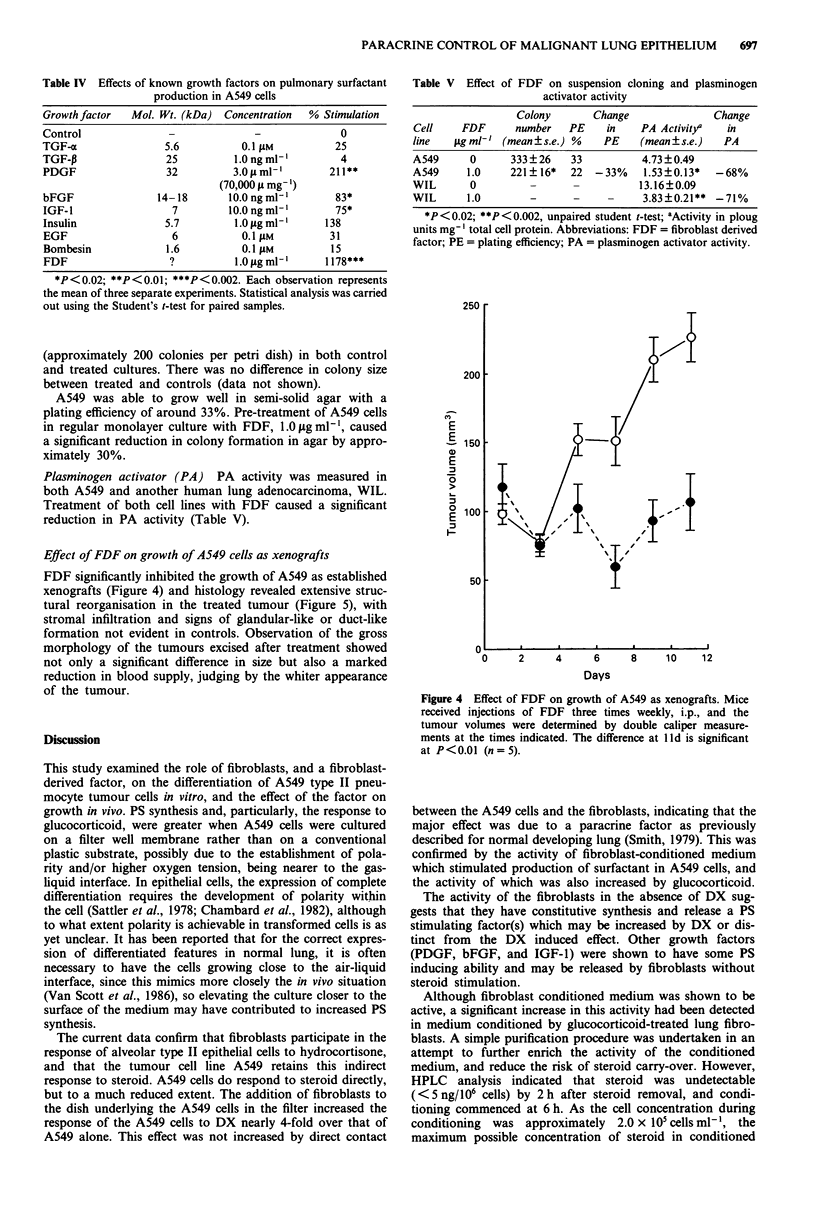
Synthesis of pulmonary surfactant (PS) is necessary for normal functioning of the lungs and its production is indicative of normal differentiated lung. The human alveolar carcinoma, A549, has been found to synthesis and secrete PS in vitro. The purpose of this study was to optimise the culture conditions for PS synthesis by A549 as well as to determine the potential role of foetal lung fibroblasts in the induction of PS by glucocorticoids. A549 cells growing in filter wells produced higher levels of PS in response to steroid, a 5-fold increase on the filter well compared to only a 1.5-fold increase when the cells were cultured on a conventional plastic substrate. A549 cells grown in filter wells responded to coculture with fibroblasts whether in direct contact or separated co-culture. A 20-fold increase in PS over control values was observed in separated steroid-treated co-cultures, suggesting the presence of a diffusible factor. A partially purified factor was isolated from fibroblast conditioned medium which was capable of inducing differentiation and other phenotypic changes in A549, namely induction of PS, reduction of plasminogen activator activity and reduction in the in vivo growth of A549 xenografts in nude mice. These results suggest that, under the correct conditions, A549 cells, although transformed, still retain the capacity to respond to differentiation-inducing signals from normal fibroblasts.
Full text
PDF

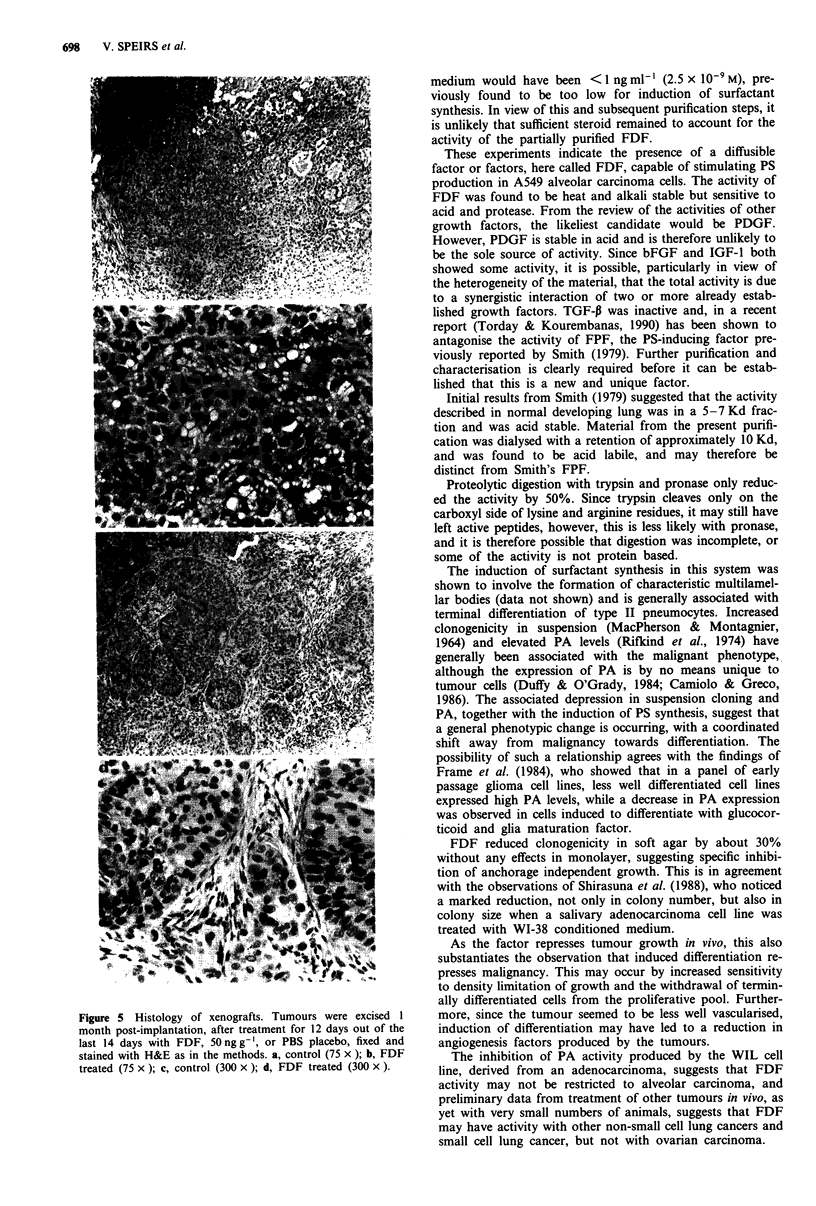

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohnert A., Hornung J., Mackenzie I. C., Fusenig N. E. Epithelial-mesenchymal interactions control basement membrane production and differentiation in cultured and transplanted mouse keratinocytes. Cell Tissue Res. 1986;244(2):413–429. doi: 10.1007/BF00219217. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Rupniak H. T., Fusenig N. E. Environmental modulation of the expression of differentiation and malignancy in six human squamous cell carcinoma cell lines. Cancer Res. 1985 Nov;45(11 Pt 2):5582–5592. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Camiolo S. M., Greco W. R. Plasminogen activator content of human tumor and adjacent normal tissue measured with fibrin and non-fibrin assays. Cancer Res. 1986 Apr;46(4 Pt 1):1788–1794. [PubMed] [Google Scholar]
- Chambard M., Gabrion J., Verrier B., Mauchamp J. Thyroid cell polarization in culture and the expression of specialized functions. Prog Clin Biol Res. 1982;91:403–411. [PubMed] [Google Scholar]
- Chen T. R. In situ detection of mycoplasma contamination in cell cultures by fluorescent Hoechst 33258 stain. Exp Cell Res. 1977 Feb;104(2):255–262. doi: 10.1016/0014-4827(77)90089-1. [DOI] [PubMed] [Google Scholar]
- Cunha G. R., Fujii H., Neubauer B. L., Shannon J. M., Sawyer L., Reese B. A. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983 Jun;96(6):1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakiew D., Tarkington M. A., Lynch J. H. Paracrine stimulation of polarized secretion from monolayers of a neoplastic prostatic epithelial cell line by prostatic stromal cell proteins. Cancer Res. 1990 Mar 15;50(6):1966–1974. [PubMed] [Google Scholar]
- Duffy M. J., O'Grady P. Plasminogen activator and cancer. Eur J Cancer Clin Oncol. 1984 May;20(5):577–582. doi: 10.1016/0277-5379(84)90001-4. [DOI] [PubMed] [Google Scholar]
- Fergusson R. J., Carmichael J., Smyth J. F. Human tumour xenografts growing in immunodeficient mice: a useful model for assessing chemotherapeutic agents in bronchial carcinoma. Thorax. 1986 May;41(5):376–380. doi: 10.1136/thx.41.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Freshney R. I., Vaughan P. F., Graham D. I., Shaw R. Interrelationship between differentiation and malignancy-associated properties in glioma. Br J Cancer. 1984 Mar;49(3):269–280. doi: 10.1038/bjc.1984.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Goerke J. Lung surfactant. Biochim Biophys Acta. 1974 Dec 16;344(3-4):241–261. doi: 10.1016/0304-4157(74)90009-4. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Cunha G. R., Wong Y. C. Influence of male genital tract mesenchymes on differentiation of Dunning prostatic adenocarcinoma. Cancer Res. 1990 Aug 1;50(15):4747–4754. [PubMed] [Google Scholar]
- Imanishi J., Hoshino S., Matsuoka H., Uemura H., Imanishi T., Tanaka A., Nishino H., Kishida T. Tumor degeneration by human embryonic fibroblasts and its enhancement by interferon. Cancer Res. 1983 Sep;43(9):4323–4326. [PubMed] [Google Scholar]
- Kédinger M., Simon-Assmann P., Alexandre E., Haffen K. Importance of a fibroblastic support for in vitro differentiation of intestinal endodermal cells and for their response to glucocorticoids. Cell Differ. 1987 Mar;20(2-3):171–182. doi: 10.1016/0045-6039(87)90431-3. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Mason R. J., Nellenbogen J., Clements J. A. Isolation of disaturated phosphatidylcholine with osmium tetroxide. J Lipid Res. 1976 May;17(3):281–284. [PubMed] [Google Scholar]
- McLean J. S., Frame M. C., Freshney R. I., Vaughan P. F., Mackie A. E., Singer I. Phenotypic modification of human glioma and non-small cell lung carcinoma by glucocorticoids and other agents. Anticancer Res. 1986 Sep-Oct;6(5):1101–1106. [PubMed] [Google Scholar]
- Neubauer B. L., Chung L. W., McCormick K. A., Taguchi O., Thompson T. C., Cunha G. R. Epithelial-mesenchymal interactions in prostatic development. II. Biochemical observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. J Cell Biol. 1983 Jun;96(6):1671–1676. doi: 10.1083/jcb.96.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podesta A. H., Mullins J., Pierce G. B., Wells R. S. The neurula stage mouse embryo in control of neuroblastoma. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7608–7611. doi: 10.1073/pnas.81.23.7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post M., Floros J., Smith B. T. Inhibition of lung maturation by monoclonal antibodies against fibroblast-pneumonocyte factor. Nature. 1984 Mar 15;308(5956):284–286. doi: 10.1038/308284a0. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Loeb J. N., Moore G., Reich E. Properties of plasminogen activators formed by neoplastic human cell cultures. J Exp Med. 1974 May 1;139(5):1317–1328. doi: 10.1084/jem.139.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D. R., Tindall D. J. Responses of NBT-II bladder carcinoma cells to conditioned medium from normal fetal urogenital sinus. Cancer Res. 1987 Jun 1;47(11):2955–2960. [PubMed] [Google Scholar]
- Sattler C. A., Michalopoulos G., Sattler G. L., Pitot H. C. Ultrastructure of adult rat hepatocytes cultured on floating collagen membranes. Cancer Res. 1978 Jun;38(6):1539–1549. [PubMed] [Google Scholar]
- Shirasuna K., Morioka S., Watatani K., Hayashido Y., Furusawa H., Sugiyama M., Okura M., Matsuya T. Growth inhibition and differentiation of human salivary adenocarcinoma cells by medium conditioned with normal human fibroblasts. Cancer Res. 1988 May 15;48(10):2819–2824. [PubMed] [Google Scholar]
- Smith B. T. Cell line A549: a model system for the study of alveolar type II cell function. Am Rev Respir Dis. 1977 Feb;115(2):285–293. doi: 10.1164/arrd.1977.115.2.285. [DOI] [PubMed] [Google Scholar]
- Smith B. T., Fletcher W. A. Pulmonary epithelial-mesenchymal interactions: beyond organogenesis. Hum Pathol. 1979 May;10(3):248–250. doi: 10.1016/s0046-8177(79)80020-9. [DOI] [PubMed] [Google Scholar]
- Smith B. T. Lung maturation in the fetal rat: acceleration by injection of fibroblast-pneumonocyte factor. Science. 1979 Jun 8;204(4397):1094–1095. doi: 10.1126/science.582216. [DOI] [PubMed] [Google Scholar]
- Taderera J. V. Control of lung differentiation in vitro. Dev Biol. 1967 Nov;16(5):489–512. doi: 10.1016/0012-1606(67)90061-9. [DOI] [PubMed] [Google Scholar]
- Torday J. S., Kourembanas S. Fetal rat lung fibroblasts produce a TGF beta homolog that blocks alveolar type II cell maturation. Dev Biol. 1990 May;139(1):35–41. doi: 10.1016/0012-1606(90)90276-o. [DOI] [PubMed] [Google Scholar]
- Van Scott M. R., Yankaskas J. R., Boucher R. C. Culture of airway epithelial cells: research techniques. Exp Lung Res. 1986;11(2):75–94. doi: 10.3109/01902148609063272. [DOI] [PubMed] [Google Scholar]
- Whur P., Magudia M., Boston J., Lockwood J., Williams D. C. Plasminogen activator in cultured Lewis lung carcinoma cells measured by chromogenic substrate assay. Br J Cancer. 1980 Aug;42(2):305–313. doi: 10.1038/bjc.1980.231. [DOI] [PMC free article] [PubMed] [Google Scholar]