Abstract
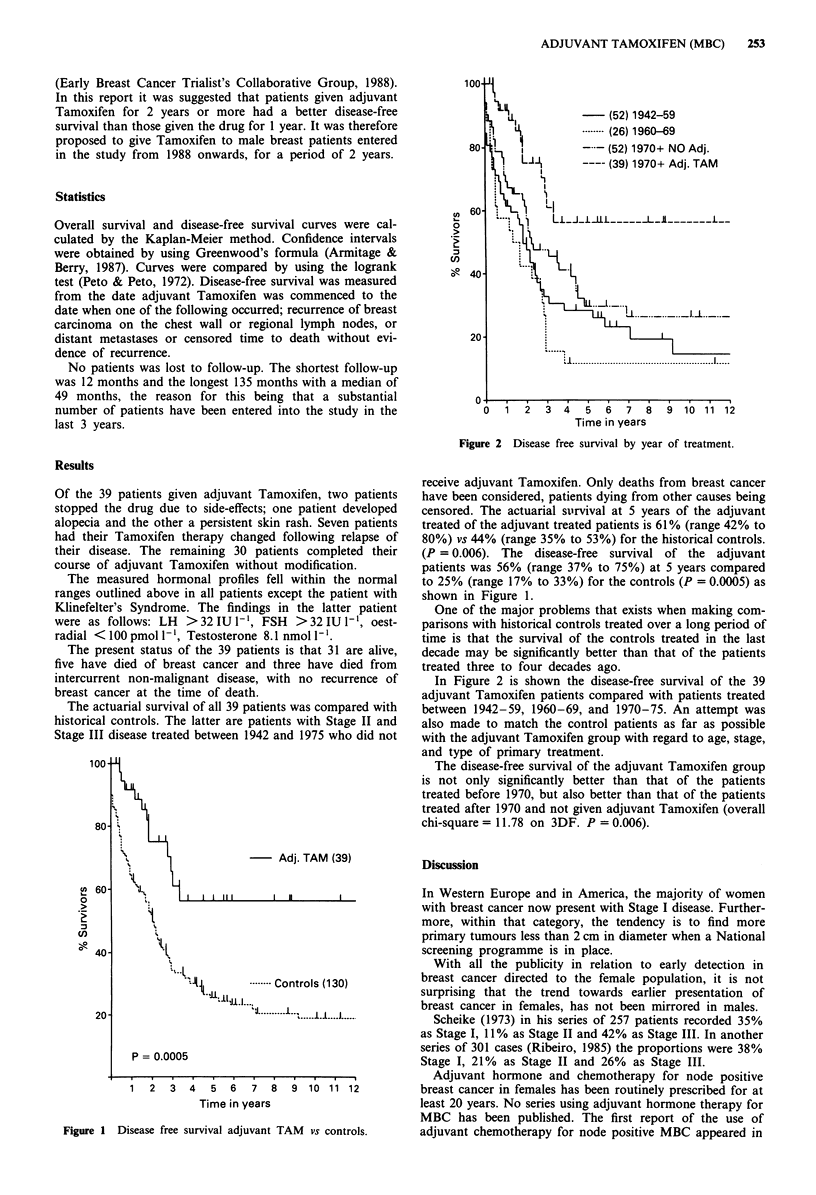
A study was started in 1976 whereby patients with Stage II and operable Stage III MBC were given adjuvant Tamoxifen for 1 year, increasing to 2 years from 1988. All patients had axillary nodal involvement. Primary treatment consisted of a radical mastectomy or simple mastectomy with radiotherapy. The rarity of the disease precluded a randomised trial. Thirty-nine patients are available for analysis at a median follow-up of 49 months. The actuarial survival of the Tamoxifen treated patients is 61% (range 42-80%) at 5 years compared to 44% (range 35-53%) for historical controls (P = 0.006). Disease-free survival was 56% (37-75%) vs 28% (17-33%) at 5 years (P = 0.005). There were no serious side-effects recorded. The conclusion from this, the first reported series on adjuvant Tamoxifen therapy for MBC, is that significant improvement in disease-free survival can be achieved with minimal upset to the patients. Recruitment to the study continues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adami H. O., Holmberg L., Malker B., Ries L. Long-term survival in 406 males with breast cancer. Br J Cancer. 1985 Jul;52(1):99–103. doi: 10.1038/bjc.1985.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley C. S., Wesley M. N., Young R. C., Lippman M. E. Adjuvant chemotherapy in males with cancer of the breast. Am J Clin Oncol. 1987 Feb;10(1):55–60. doi: 10.1097/00000421-198702000-00013. [DOI] [PubMed] [Google Scholar]
- Baker H. W., Burger H. G., de Kretser D. M., Hudson B., O'Connor S., Wang C., Mirovics A., Court J., Dunlop M., Rennie G. C. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976 Jul;5(4):349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Barnes D. M., Ribeiro G. G., Skinner L. G. Two methods for measurement of oestradiol-17 beta and progesterone receptors in human breast cancer and correlation with response treatment. Eur J Cancer. 1977 Oct;13(10):1133–1143. doi: 10.1016/0014-2964(77)90012-3. [DOI] [PubMed] [Google Scholar]
- Crichlow R. W. Breast cancer in men. Semin Oncol. 1974 Jun;1(2):145–152. [PubMed] [Google Scholar]
- Fisher B., Costantino J., Redmond C., Poisson R., Bowman D., Couture J., Dimitrov N. V., Wolmark N., Wickerham D. L., Fisher E. R. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989 Feb 23;320(8):479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- Ribeiro G. G., Phillips H. V., Skinner L. G. Serum oestradiol-17 beta, testosterone, luteinizing hormone and follicle-stimulating hormone in males with breast cancer. Br J Cancer. 1980 Mar;41(3):474–477. doi: 10.1038/bjc.1980.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro G. G. Tamoxifen in the treatment of male breast carcinoma. Clin Radiol. 1983 Nov;34(6):625–628. doi: 10.1016/s0009-9260(83)80408-5. [DOI] [PubMed] [Google Scholar]
- Ribeiro G. Male breast carcinoma--a review of 301 cases from the Christie Hospital & Holt Radium Institute, Manchester. Br J Cancer. 1985 Jan;51(1):115–119. doi: 10.1038/bjc.1985.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheike O. Male breast cancer. 5. Clinical manifestations in 257 cases in Denmark. Br J Cancer. 1973 Dec;28(6):552–561. doi: 10.1038/bjc.1973.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap H. Y., Tashima C. K., Blumenschein G. R., Eckles N. E. Male breast cancer: a natural history study. Cancer. 1979 Aug;44(2):748–754. doi: 10.1002/1097-0142(197908)44:2<748::aid-cncr2820440248>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]


