Abstract
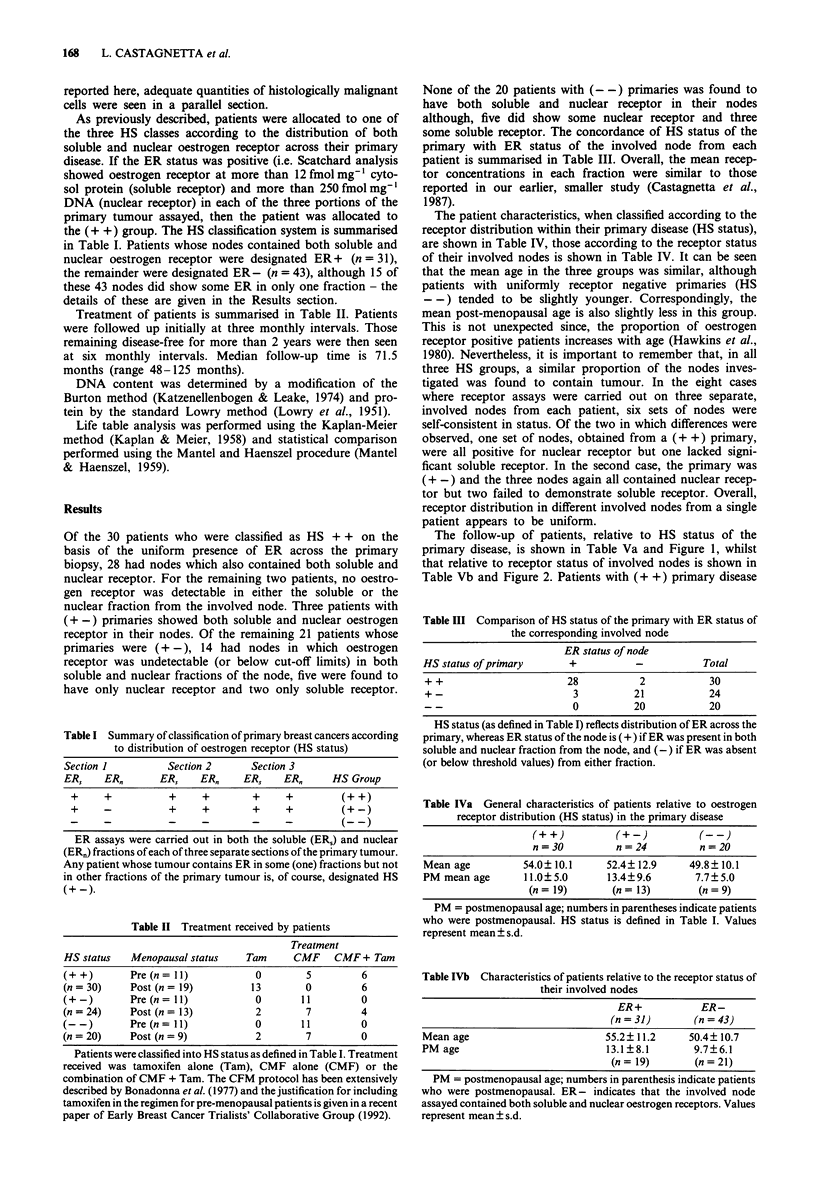
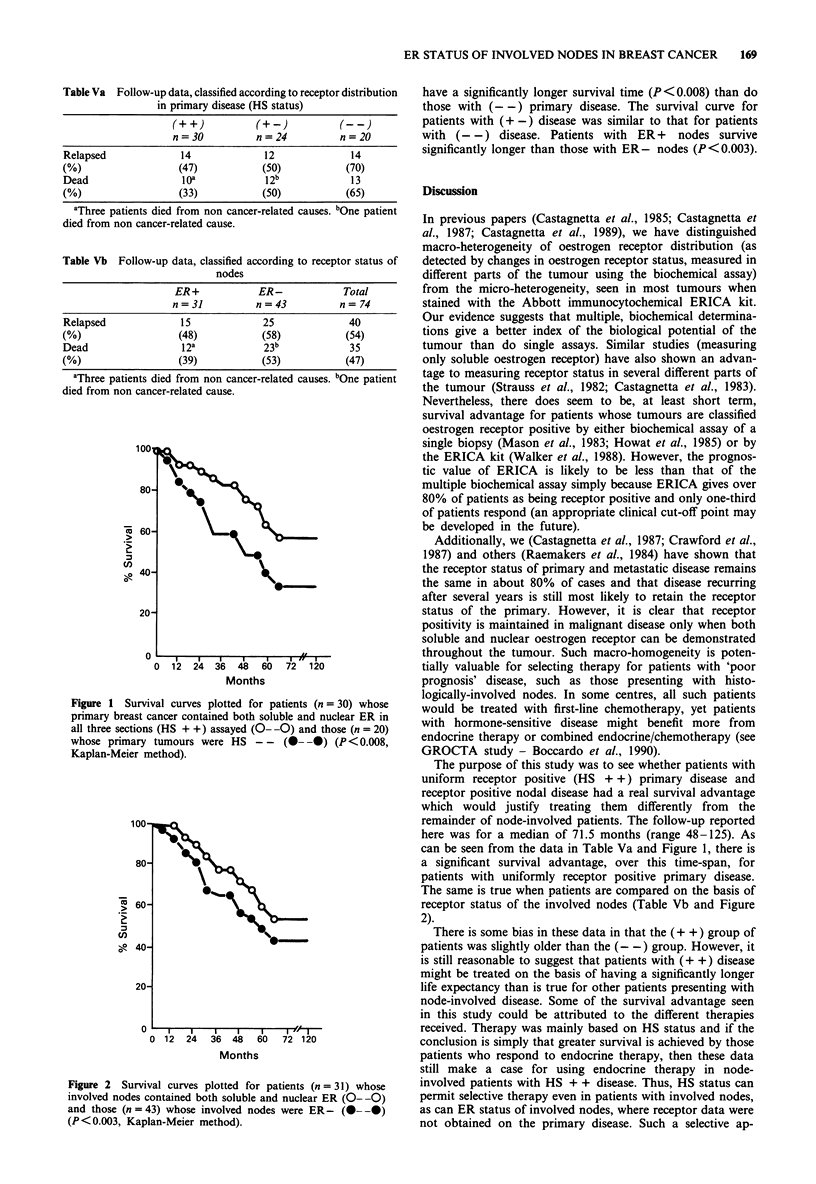
Nodal involvement is accepted as the best single marker of prognosis in breast cancer. However, there is little information on the sub-division of node-positive patients according to the oestrogen receptor status of the nodal tissue. We have previously reported (Eur. J. Ca. 1987, 23, 31) that, in almost all cases, involved nodes are only oestrogen receptor positive (ER+) in patients whose primary tumours are uniformly ER+. This paper presents clinical follow-up on a larger group of patients with node positive breast cancer. For each patient, both soluble and nuclear receptor concentrations were determined in three separate parts of the primary tumour and in at least one involved node (we have previously defined tumours which contained ER in all six fractions of the primary as HS++, those lacking receptor in some fractions as HS+- and wholly receptor negative tumours as HS--). Median follow-up time was 71.5 months. As expected, patients whose tumours were HS++ had a significant (P less than 0.008) survival advantage. More importantly, patients with ER in both the soluble and nuclear fractions of their involved nodes survived significantly (P less than 0.003) longer than those with ER- nodes. Thus, full oestrogen receptor status of involved nodes will give sufficient prognostic information when adequate primary tissue is not available.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boccardo F., Rubagotti A., Bruzzi P., Cappellini M., Isola G., Nenci I., Piffanelli A., Scanni A., Sismondi P., Santi L. Chemotherapy versus tamoxifen versus chemotherapy plus tamoxifen in node-positive, estrogen receptor-positive breast cancer patients: results of a multicentric Italian study. Breast Cancer Adjuvant Chemo-Hormone Therapy Cooperative Group. J Clin Oncol. 1990 Aug;8(8):1310–1320. doi: 10.1200/JCO.1990.8.8.1310. [DOI] [PubMed] [Google Scholar]
- Bonadonna G., Rossi A., Valagussa P., Banfi A., Veronesi U. The CMF program for operable breast cancer with positive axillary nodes. Updated analysis on the disease-free interval, site of relapse and drug tolerance. Cancer. 1977 Jun;39(6 Suppl):2904–2915. doi: 10.1002/1097-0142(197706)39:6<2904::aid-cncr2820390677>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Castagnetta L., Lo Casto M., Mercadante T., Polito L., Cowan S., Leake R. E. Intra-tumoural variation of oestrogen receptor status in endometrial cancer. Br J Cancer. 1983 Feb;47(2):261–267. doi: 10.1038/bjc.1983.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagnetta L., Traina A., Di Carlo A., Latteri A. M., Carruba G., Leake R. E. Heterogeneity of soluble and nuclear oestrogen receptor status of involved nodes in relation to primary breast cancer. Eur J Cancer Clin Oncol. 1987 Jan;23(1):31–35. doi: 10.1016/0277-5379(87)90415-9. [DOI] [PubMed] [Google Scholar]
- Crawford D. J., Cowan S., Fitch R., Smith D. C., Leake R. E. Stability of oestrogen receptor status in sequential biopsies from patients with breast cancer. Br J Cancer. 1987 Aug;56(2):137–140. doi: 10.1038/bjc.1987.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howat J. M., Harris M., Swindell R., Barnes D. M. The effect of oestrogen and progesterone receptors on recurrence and survival in patients with carcinoma of the breast. Br J Cancer. 1985 Feb;51(2):263–270. doi: 10.1038/bjc.1985.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Leake R. E. Distribution of the oestrogen-induced protein and of total protein between endometrial and myometrial fractions of the immature and mature rat uterus. J Endocrinol. 1974 Dec;63(3):439–449. doi: 10.1677/joe.0.0630439. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee K., Tanaka M., Kanamaru H., Hashimura T., Yamamoto I., Konishi J., Kuze F. In vitro antagonism between cisplatin and vinca alkaloids. Br J Cancer. 1989 Jan;59(1):36–41. doi: 10.1038/bjc.1989.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Mason B. H., Holdaway I. M., Mullins P. R., Yee L. H., Kay R. G. Progesterone and estrogen receptors as prognostic variables in breast cancer. Cancer Res. 1983 Jun;43(6):2985–2990. [PubMed] [Google Scholar]
- Pertschuk L. P., Eisenberg K. B., Carter A. C., Feldman J. G. Heterogeneity of estrogen binding sites in breast cancer: morphologic demonstration and relationship to endocrine response. Breast Cancer Res Treat. 1985;5(2):137–147. doi: 10.1007/BF01805987. [DOI] [PubMed] [Google Scholar]
- Raemaekers J. M., Beex L. V., Koenders A. J., Pieters G. F., Smals A. G., Benraad T. J., Kloppenborg P. W. Concordance and discordance of estrogen and progesterone receptor content in sequential biopsies of patients with advanced breast cancer: relation to survival. Eur J Cancer Clin Oncol. 1984 Aug;20(8):1011–1018. doi: 10.1016/0277-5379(84)90102-0. [DOI] [PubMed] [Google Scholar]
- Silfverswärd C., Skoog L., Humla S., Gustafsson S. A., Nordenskjöld B. Intratumoral variation of cytoplasmic and nuclear estrogen receptor concentrations in human mammary carcinoma. Eur J Cancer. 1980 Jan;16(1):59–65. doi: 10.1016/0014-2964(80)90108-5. [DOI] [PubMed] [Google Scholar]
- Straus M. J., Moran R., Muller R. E., Wotiz H. H. Estrogen receptor heterogeneity and the relationship between estrogen receptor and the tritiated thymidine labeling index in human breast cancer. Oncology. 1982;39(4):197–200. doi: 10.1159/000225636. [DOI] [PubMed] [Google Scholar]
- Walker K. J., Bouzubar N., Robertson J., Ellis I. O., Elston C. W., Blamey R. W., Wilson D. W., Griffiths K., Nicholson R. I. Immunocytochemical localization of estrogen receptor in human breast tissue. Cancer Res. 1988 Nov 15;48(22):6517–6522. [PubMed] [Google Scholar]


