Abstract
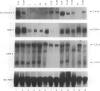
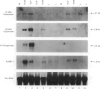
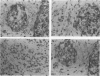
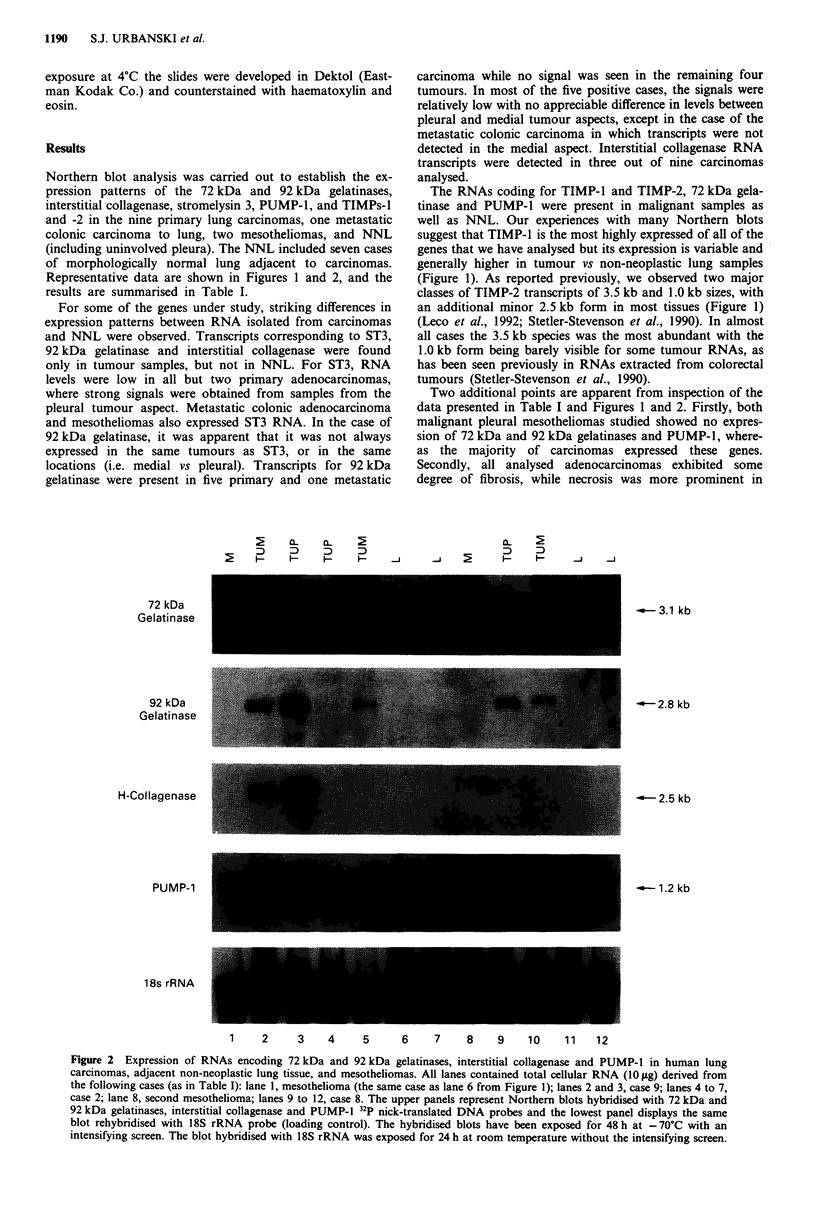
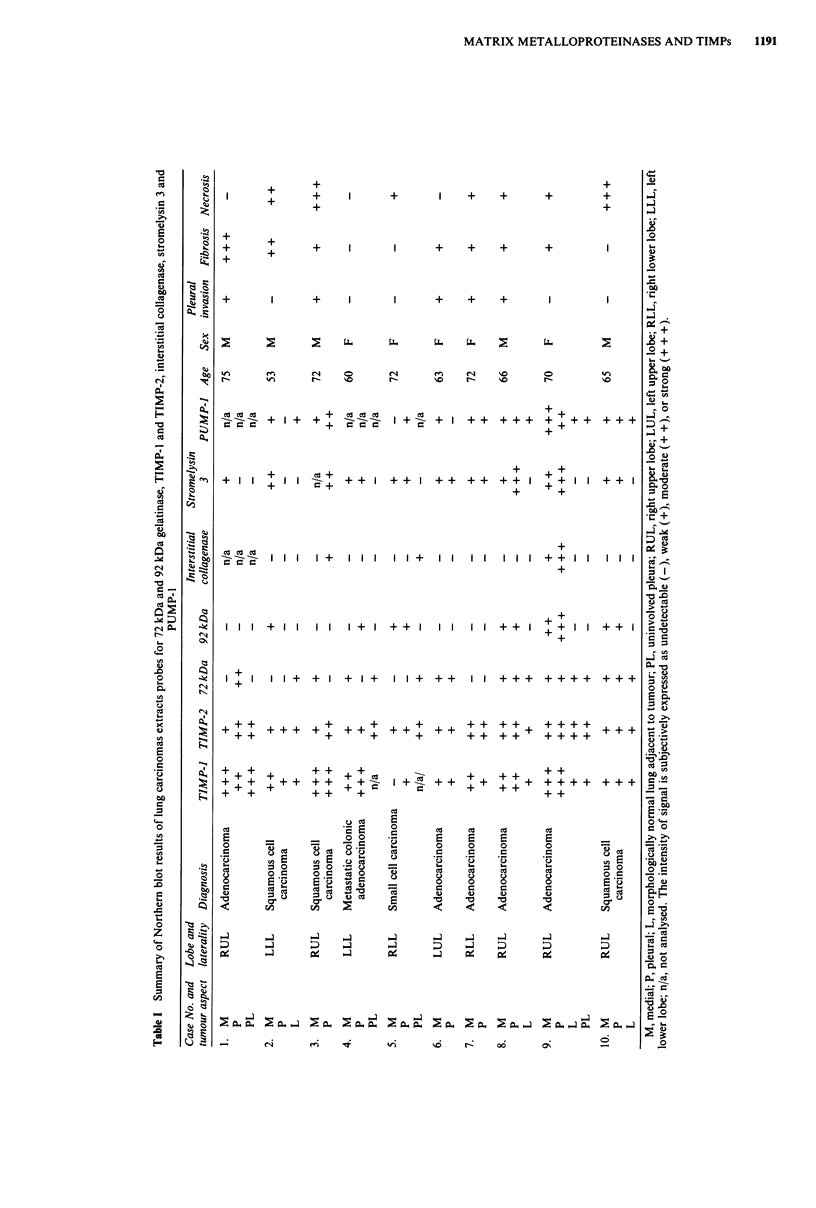
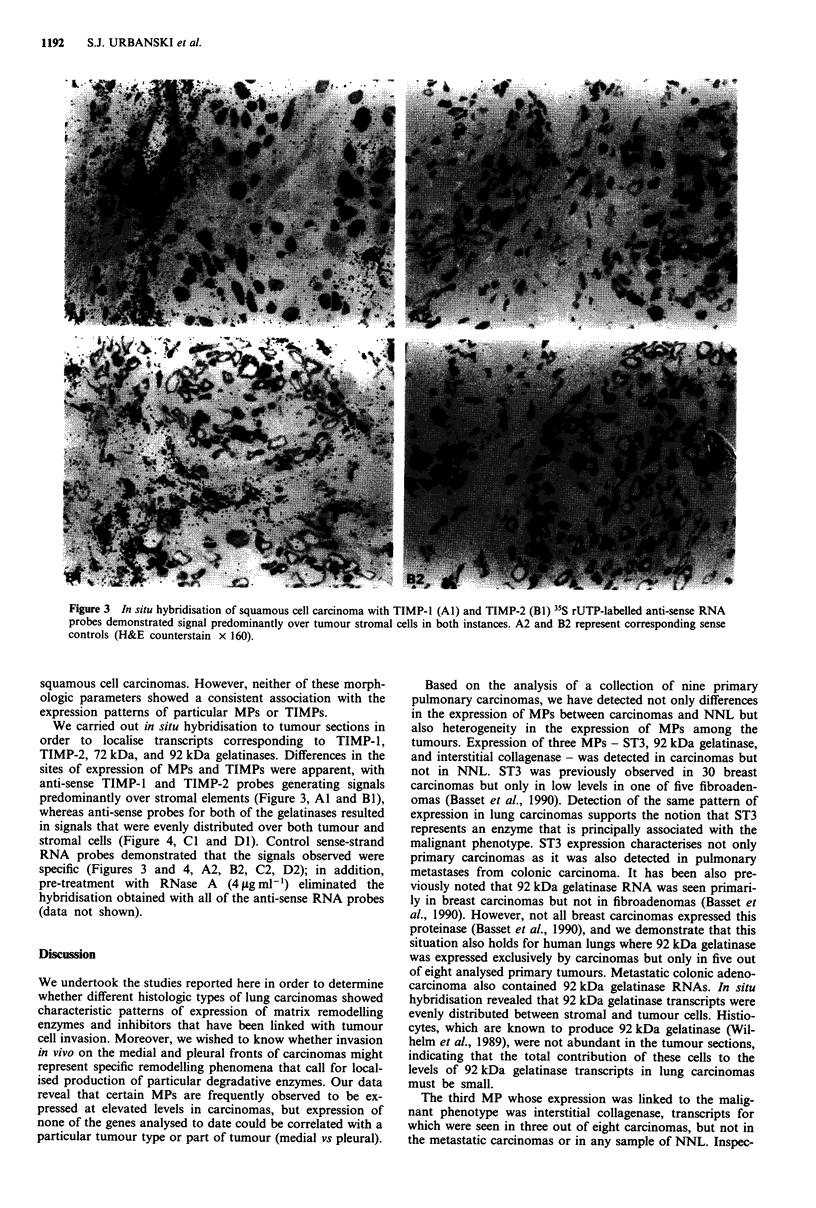
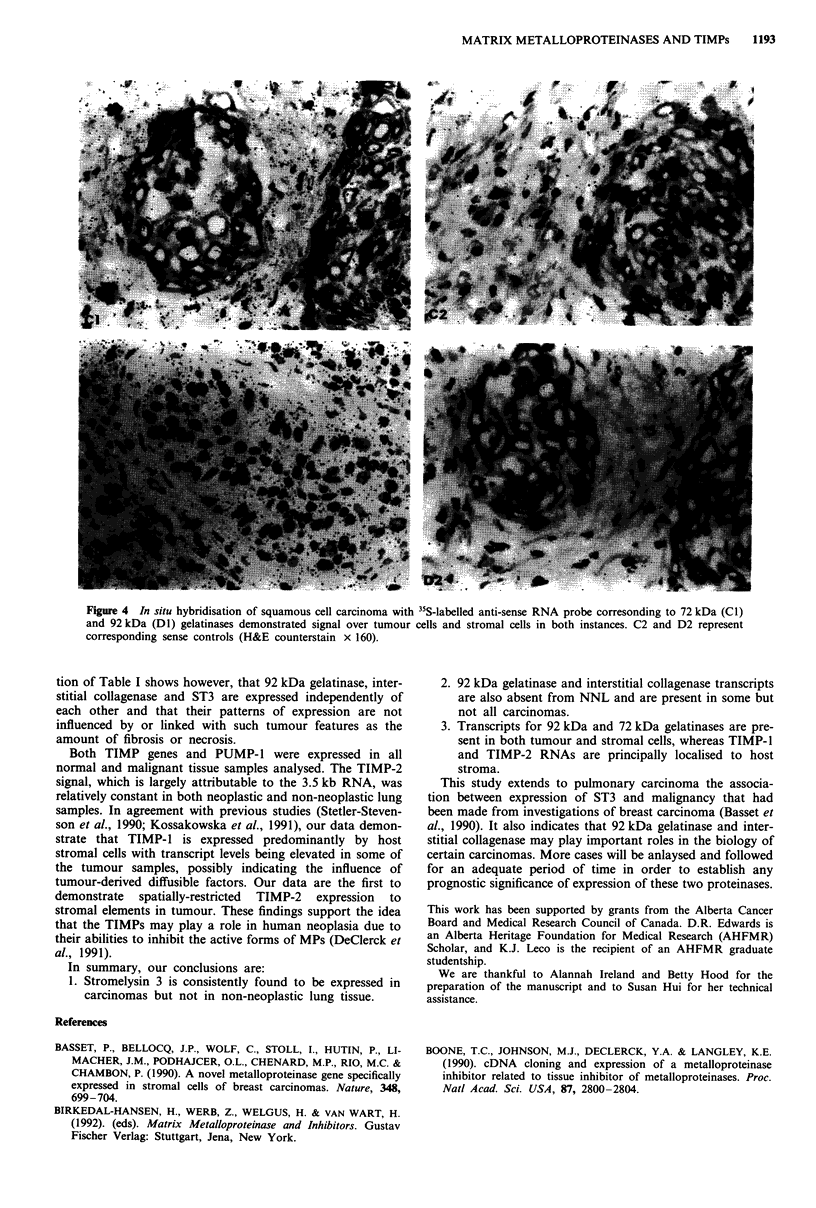
Nine primary pulmonary carcinomas, one metastatic carcinoma, and two malignant pleural mesotheliomas have been analysed for the expression at the mRNA level of metalloproteinases (MPs) and tissue inhibitors of MPs (TIMPs). In situ hybridisation showed TIMP-1 and TIMP-2 transcripts predominantly over tumour stroma and gelatinases evenly distributed over both stromal and tumour cells. While both TIMP-1 and TIMP-2 were expressed in non-neoplastic lungs (NNL) as well as in carcinomas, stromelysin 3 (ST3), 92 kDa gelatinase and interstitial collagenase were expressed only by carcinomas. Expression of these MPs by carcinomas was independent of histologic type and such tumour features as fibrosis or necrosis. The consistent expression of ST3 by all of the carcinomas examined and absence of its expression in NNL indicates that ST3 production is likely associated with the malignant phenotype. However, since 92 kDa gelatinase and interstitial collagenase transcripts were found in some but not all tumour samples, their expression is not a uniform feature of pulmonary carcinomas. The possible prognostic significance of the expression of the latter two enzymes by carcinomas remains to be established.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basset P., Bellocq J. P., Wolf C., Stoll I., Hutin P., Limacher J. M., Podhajcer O. L., Chenard M. P., Rio M. C., Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990 Dec 20;348(6303):699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Boone T. C., Johnson M. J., De Clerck Y. A., Langley K. E. cDNA cloning and expression of a metalloproteinase inhibitor related to tissue inhibitor of metalloproteinases. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2800–2804. doi: 10.1073/pnas.87.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. D., Levy A. T., Margulies I. M., Liotta L. A., Stetler-Stevenson W. G. Independent expression and cellular processing of Mr 72,000 type IV collagenase and interstitial collagenase in human tumorigenic cell lines. Cancer Res. 1990 Oct 1;50(19):6184–6191. [PubMed] [Google Scholar]
- Circolo A., Welgus H. G., Pierce G. F., Kramer J., Strunk R. C. Differential regulation of the expression of proteinases/antiproteinases in fibroblasts. Effects of interleukin-1 and platelet-derived growth factor. J Biol Chem. 1991 Jul 5;266(19):12283–12288. [PubMed] [Google Scholar]
- Collier I. E., Wilhelm S. M., Eisen A. Z., Marmer B. L., Grant G. A., Seltzer J. L., Kronberger A., He C. S., Bauer E. A., Goldberg G. I. H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem. 1988 May 15;263(14):6579–6587. [PubMed] [Google Scholar]
- DeClerck Y. A., Yean T. D., Lu H. S., Ting J., Langley K. E. Inhibition of autoproteolytic activation of interstitial procollagenase by recombinant metalloproteinase inhibitor MI/TIMP-2. J Biol Chem. 1991 Feb 25;266(6):3893–3899. [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Marmer B. L., Grant G. A., Eisen A. Z., Wilhelm S., He C. S. Human 72-kilodalton type IV collagenase forms a complex with a tissue inhibitor of metalloproteases designated TIMP-2. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8207–8211. doi: 10.1073/pnas.86.21.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossakowska A. E., Urbanski S. J., Edwards D. R. Tissue inhibitor of metalloproteinases-1 (TIMP-1) RNA is expressed at elevated levels in malignant non-Hodgkin's lymphomas. Blood. 1991 Jun 1;77(11):2475–2481. [PubMed] [Google Scholar]
- Leco K. J., Hayden L. J., Sharma R. R., Rocheleau H., Greenberg A. H., Edwards D. R. Differential regulation of TIMP-1 and TIMP-2 mRNA expression in normal and Ha-ras-transformed murine fibroblasts. Gene. 1992 Aug 15;117(2):209–217. doi: 10.1016/0378-1119(92)90731-4. [DOI] [PubMed] [Google Scholar]
- Matrisian L. M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990 Apr;6(4):121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantin B., Murphy G., Breathnach R. Pump-1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochemistry. 1989 Jun 27;28(13):5327–5334. doi: 10.1021/bi00439a004. [DOI] [PubMed] [Google Scholar]
- Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986 Aug 7;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Brown P. D., Onisto M., Levy A. T., Liotta L. A. Tissue inhibitor of metalloproteinases-2 (TIMP-2) mRNA expression in tumor cell lines and human tumor tissues. J Biol Chem. 1990 Aug 15;265(23):13933–13938. [PubMed] [Google Scholar]
- Stetler-Stevenson W. G., Krutzsch H. C., Liotta L. A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J Biol Chem. 1989 Oct 15;264(29):17374–17378. [PubMed] [Google Scholar]
- Whitham S. E., Murphy G., Angel P., Rahmsdorf H. J., Smith B. J., Lyons A., Harris T. J., Reynolds J. J., Herrlich P., Docherty A. J. Comparison of human stromelysin and collagenase by cloning and sequence analysis. Biochem J. 1986 Dec 15;240(3):913–916. doi: 10.1042/bj2400913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S. M., Collier I. E., Marmer B. L., Eisen A. Z., Grant G. A., Goldberg G. I. SV40-transformed human lung fibroblasts secrete a 92-kDa type IV collagenase which is identical to that secreted by normal human macrophages. J Biol Chem. 1989 Oct 15;264(29):17213–17221. [PubMed] [Google Scholar]