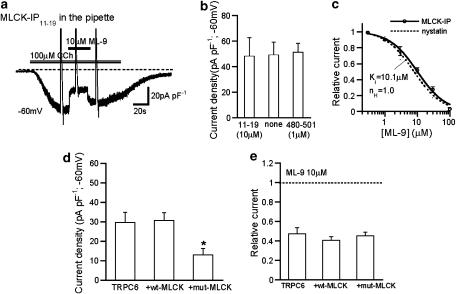
Figure 4.
Intracellular perfusion of MLCK-inhibitory peptides and dominant negative inhibition of MLCK did not affect the inhibitory efficacy of ML-9 on ITRPC6. Conventional whole-cell recording at −60 mV. To reduce the degree of desensitization, Ca2+-free rather than normal external solution was used as the bathing solution. (a) Representative trace for the extracellular ML-9 (10 μM) effects on ITRPC6 in the presence of 10 μM MLCK IP11–19 peptide in the pipette. (b) Summary of the effects of 10 μM MLCK IP11–19 and 1 μM MLCK IP480–501 on ITRPC6 density (n=7–11). (c) ML-9 concentration–inhibition curves for ITRPC6 with (solid) and without (nystatin-perforated recording; dotted; taken from Figure 1b) inclusion of MLCK inhibitory peptides (10 μM MLCK IP11–19 or 1 μM MLCK IP480–501). The solid curve is drawn according to the best fit of the data points with Hill equation (see Methods). IC50=10.1 μM; nH=1.0 (n=4–6). There was no statistically significant difference between two curves with ANOVA. (d and e) Influence of overexpression of wild type (wt-MLCK) or a dominant-negative mutant of MLCK (mut-MLCK) on current density of ITRPC6 (d; n=15–18) and inhibitory efficacy of ML-9 for ITRPC6 (e; n=8–11). (d) *P<0.05 from other columns with ANOVA and Bonferroni's t-test. ANOVA, analysis of variance; IP, inhibitory peptides; ML-9, [1-(5-chloronaphthalene-1-sulphonyl)homopiperazine, HCl]; MLCK, myosin light chain kinase.