Figure 1.
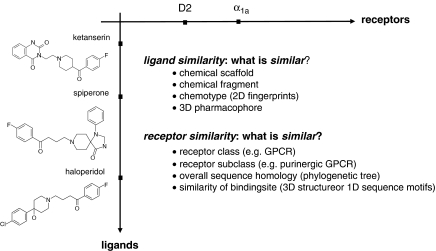
The similarity of ligands and receptors can be defined using different methods or ‘descriptors'. The similarity of ligands (for example, ketanserin, spiperone, haloperidol) can be defined either by comparing the chemical scaffold or by looking for identical structural fragments. In addition, several other descriptors (for example, 2D fingerprints, 3D pharmacophores, and so on) and metrics for comparison have been found to be relevant when defining similarity of biologically active molecules. How to define the similarity of receptors (for example, α1a adrenergic receptor and D2 dopamine receptor)? Proteins that belong to the same target family or class, for example, the family of GPCRs, can be considered as similar. A more detailed classification level is defining two receptors as being similar, if they bind the same class of ligands, for example, peptides. This method groups GPCRs in different subclasses such as chemokine receptors, peptide-binding GPCRs or purinergic GPCRs. Another classification level is based on sequence similarity of the receptors. And finally, a further relevant viewpoint for a chemogenomics-driven classification approach is the comparison of two receptors based on the similarity of their putative ligand-binding sites, regardless of their phylogenetic relationship. This is the best indication that a pair of receptors would bind similar ligands.