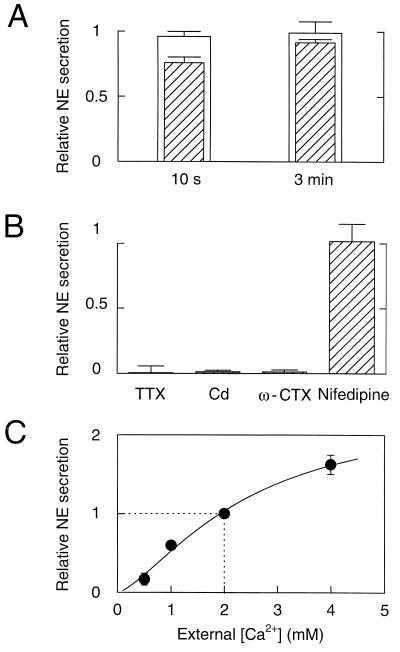
Figure 2.
Recovery, pharmacology, and Ca2+ dependence of NE secretion. (A) Recovery of secretion. NE secretion was induced by a 1-s, high K+ deplarization as in Fig. 1B or by electrical stimuli as in Fig. 1C. NE secretion was measured by integrating electrochemical records. Relative NE secretion was calculated as the ratio of NE secretion induced by a second stimulus relative to the first stimulus. Intervals between two stimuli were 10 s or 3 min. White bars denote secretion by electrical stimulation (n = 4), and hatched bars denote secretion by K+ stimulation (n = 4). (B) NE secretion measured in the absence and presence of Na+ and Ca2+ channel blockers. Perfusion of solutions containing blockers started 15 s before electrical stimuli and continued during the recording, except ω-conotoxin GVIA, which was perfused for 1 min. NE secretion relative to controls (see Materials and Methods) was measured in the presence of 300 nM of TTX (0.1 ± 5.2% compared with control value) (n = 6), 100 μM Cd (1.3 ± 1.1%) (n = 10), 1 μM ω-conotoxin GVIA (1.2 ± 1.5%) (n = 5), and 2 μM nifedipine (102 ± 13.5%) (n = 4). (C) Secretion at different external Ca2+ concentrations ([Ca2+]o). NE secretion was induced by electrical stimulation. For [Ca2+]o of 2 mM or lower, MgCl2 was added to make the total divalent concentration 4 mM. NE secretion was normalized to that at 2 mM [Ca2+]o, which was the standard concentration in all other experiments. Data are from 13 cell clusters. The concentration–secretion response was fitted with the equation: relative NE secretion = Max/{1 + (K0.5/[Ca2+]o)a}, where Max =2.41, K0.5 = 2.43 mM Ca2+, and a = 1.43.