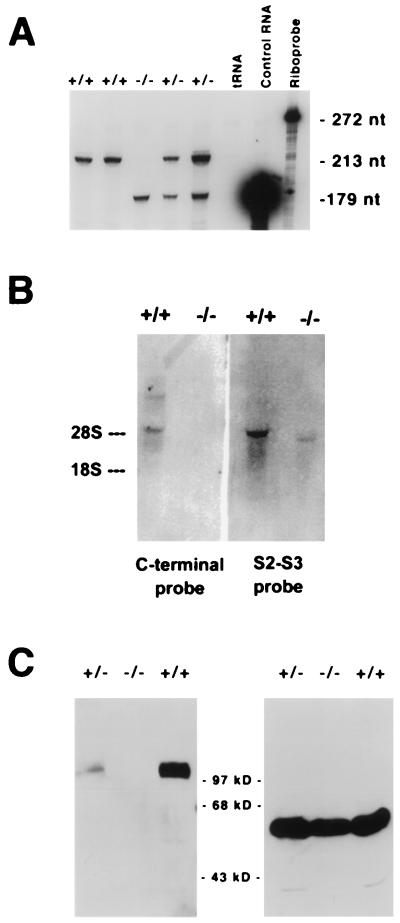
Figure 2.
(A) RNase protection assay. The targeted allele is transcribed at approximately the same level as the functional, nontargeted allele. Ten micrograms of total brain RNA was hybridized to an excess of 32P-labeled probe (272 nt in length) specific for Kv3.1 mRNA. Protected transcripts of the normal allele yielded a fragment of 213 nt after digestion with RNase A and RNase T1; transcripts of the neo cassette-interrupted allele yielded a 179-nt fragment. (B) Northern blot analysis of brain RNA. The targeted allele is transcribed but uses a different polyadenylation site. Two different radioactive probes directed either to the C-terminal end of the coding region of Kv3.1a and -b mRNA or to the S2–S3 region (upstream of the insertion site of the neomycin cassette) were used. The C-terminal probe did not hybridize to the RNA transcribed from the targeted allele; in contrast, the S2-S3 probe identified a Kv3.1-specific RNA that was shorter than the normal Kv3.1b mRNA. The shorter RNA is presumably terminated at the polyadenylation site introduced by the neomycin cassette. (C) Kv3.1 is not expressed in brain of Kv3.1−/− mutants. Western blot analysis performed on brain extract from a Kv3.1+/+ wild type showed a band migrating with an apparent molecular weight of 100 kDa (Left). Extracts from Kv3.1+/− brains showed the same band but at lower intensity. No Kv3.1 protein could be detected in extracts from homozygous Kv3.1−/− null mutant brains. A mAb specific for α2-tubulin was used as a control to monitor comparable amounts of protein on the gel (Right).