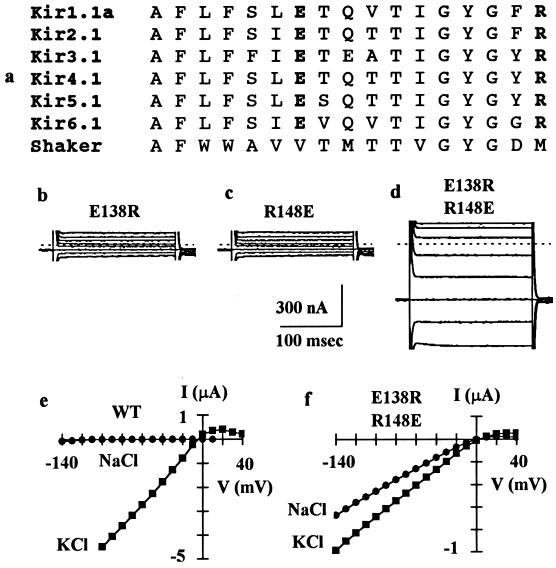
Figure 1.
Functional interactions between a pair of pore loop residues. (a) Alignment of the H5 pore loop amino acid sequences from members of six subfamilies of inwardly rectifying K+ channels. See Doupnik et al. (3) for nomenclature. The IRK1 channel used in this study corresponds to Kir2.1. The amino acid sequence corresponds to number 132–148. The H5 region sequence of the voltage-gated Shaker K+ channel is also shown for comparison. (b–d) Whole-cell currents recorded by two-electrode voltage-clamp from oocytes injected with cRNA for E138R (b), R148E (c), or E138R/R148E (d). Currents were elicited by 200-ms voltage steps to −100 to +40 mV in 20-mV increments from a holding potential of −60 mV and are not leak-subtracted. The dashed line indicates zero current level. The current amplitude at −100 mV was 111 ± 7 nA (mean ± SEM, n = 11) for E138R, 119 ± 7 nA (n = 11) for R148E, and 671 ± 27 nA (n = 20) for E138R/R148E. Oocytes injected with cRNAs for E138D, E138Q, E138C, R148K, R148H, or R148C, like oocytes injected with cRNAs for E138R or R148E, produced currents not significantly different from uninjected oocytes, which had a basal current of 116 ± 21 nA (n = 21) at −100 mV. (e–f) Current–voltage relations in 90 mM external KCl or NaCl for the wild-type IRK1 channel or mutant E138R/R148E channel. Current was measured at the end of a 200-ms test pulse and was leak-subtracted. For the wild-type channel, the reversal potential was −5.9 ± 0.3 mV (mean ± SEM, n = 9) in KCl and −103 ± 3 mV (n = 9) in NaCl. For the mutant channel, the reversal potential was −4.8 ± 1.0 mV (n = 11) in KCl and −7.2 ± 1.2 mV (n = 11) in NaCl.