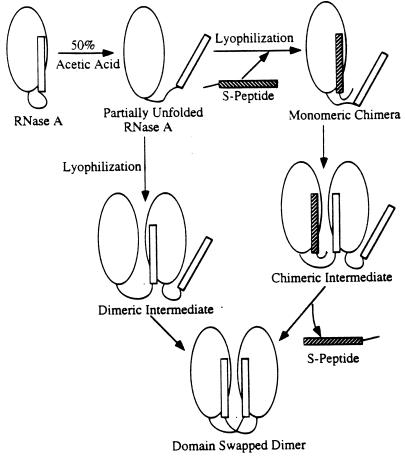
Figure 4.
Schematic illustration of competition of the S-peptide (residues 1–20) with the formation of the domain-swapped RNase A dimer. At low concentrations of the S-peptide, the S-peptide binds to a partially unfolded RNase A monomer (Top Center) and displaces its N-terminal helix, creating a monomeric chimera (Top Right), which has the potential to bind to another partially unfolded monomer to form an unstable chimeric intermediate (Right Center). Thus, low concentrations of the S-peptide might favor formation of the domain-swapped dimer. At higher concentrations of the S-peptide, most RNase A monomers are occupied by the S-peptide, preventing the formation of the chimeric intermediate (Right Center) or the dimeric intermediate (Left Center), thus diminishing the yield of the domain-swapped dimer.