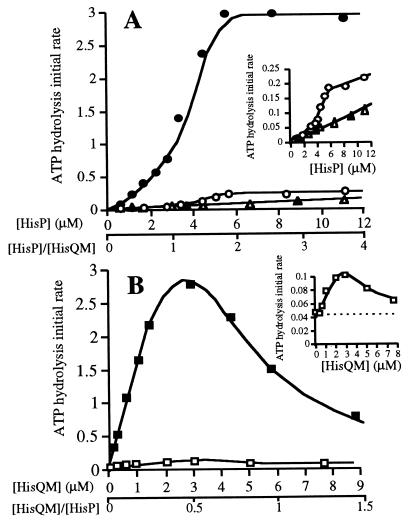
Figure 4.
Dependence of ATPase activity on HisQM and HisP concentrations. The initial rate of ATP hydrolysis was measured as described in the legend to Fig. 2, in the absence (open symbols) or presence of 18 μM l-histidine-liganded HisJ (solid symbols). The activity is expressed as nmol Pi released per min per 10 μl sample, containing various concentrations of pure HisP(his6) and/or HisQ, as indicated. All ATPase assays involving membranes were corrected for a low level contaminating ATPase activity that is present in HisQM membranes. (A) Varying amounts of soluble HisP(his6) (0–12 μM) were incubated either with HisQM membranes containing 3 μM HisQM (○ and •) or in the absence of membranes (▵ and ▴). (B) Varying amounts of HisQM membranes (containing 0–12 μM HisQM) incubated with 6 μM of soluble HisP(his6) (□ and ▪). The dotted line is drawn through the level of activity of 6 μM pure soluble HisP(his6). Both insets are enlargements of the respective data from samples with low activity. In this experiment no ultracentrifugation to separate the bound and free HisP was performed, because the ATPase activity HisP(his6) at such low concentrations in the absence of HisQM does not interfere with the assay, also shown by the fact that similar results were obtained if the reassembly mixture containing nmolar amounts of HisP(his6) were centrifuged prior to analysis.