Abstract
Multi-functional bilayer polymeric coatings are prepared with both controlled nitric oxide (NO) release and surface-bound active thrombomodulin (TM) alone or in combination with immobilized heparin. The outer-layer is made of CarboSil, a commercially available copolymer of silicone rubber (SR) and polyurethane (PU). The CarboSil is either carboxylated or aminated via an allophanate reaction with a diisocyanate compound followed by a urea-forming reaction between the generated isocyanate group of the polymer and the amine group of an amino acid (glycine), an oligopeptide (triglycine) or a diamine. The carboxylated CarboSil can then be used to immobilize TM through the formation of an amide bond between the surface carboxylic acid groups and the lysine residues of TM. Aminated CarboSil can also be employed to initially couple heparin to the surface, and then the carboxylic acid groups on heparin can be further used to anchor TM. Both surface-bound TM and heparin’s activity are evaluated by chromogenic assays and found to be at clinically significant levels. The underlying NO release layer is made with another commercial SR-PU copolymer (PurSil) mixed with a lipophilic NO donor (N-diazeniumdiolated dibutylhexanediamine (DBHD/N2O2)). The NO release rate can be tuned by changing the thickness of top coatings, and the duration of NO release at physiologically relevant levels can be as long as 2 weeks. The combination of controlled NO release as well as immobilized active TM and heparin from/on the same polymeric surface mimics the highly thromboresistant endothelium layer. Hence, such multifunctional polymer coatings should provide more blood-compatible surfaces for biomedical devices.
1. Introduction
During the past century, the use of cardiovascular and other blood contacting biomedical devices has evolved from a mere dream to a widely adopted practice, including the use of heart valves, vascular grafts, stents, extracorporeal circuits and membrane oxygenators [1, 2]. However, a lingering issue is the potential of thrombus formation and/or platelet activation at the blood/device interface owing to the lack of hemocompatibility of the polymeric materials employed to fabricate such devices [3].
Platelet adhesion/activation and thrombus formation do not readily occur on the surface of a healthy endothelium that lines the inner walls of all blood vessels. The excellent thromboresistancy of endothelial cells (ECs) is largely attributed to both secreted agents (nitric oxide (NO) [4], prostacyclin (PGI2) [5], plasminogen [6], antithrombin III (AT III) [7]) and membrane-bound species (heparan sulphate (HS) [8], thrombomodulin (TM) [9]). Both NO and PGI2 have long been recognized for their anti-platelet activity, while heparin [10] (an HS analogue) and TM are well known for their anticoagulant function.
Based on knowledge of which species contribute to the exceptional biocompatibility of the EC, efforts have been made to utilize such agents to create more hemocompatible surfaces. Some examples include NO-release [11], heparin-bound [12] or TM-immobilized [2] polymeric coatings. However, their potency in preventing clot formation may be limited by the fact that such surfaces only possess partial thromboresistance (i.e., either only anti-platelet or only anticoagulant activity present). A surface that integrates both anti-platelet and anticoagulant agents would seem most promising in that these agents can work synergistically to achieve true thromboresistance. Recently, the first polymeric coating that combines both NO release and surface-bound biologically active heparin was reported as an initial effort toward this goal [13].
Thrombomodulin is named after its bioactivity as a ‘thrombin modulator’ [14]. It is a transmembrane protein with a molecular weight of 74 kDa. The mature TM molecule consists of 557 amino acid residues and usually has a chondroitin sulfate moiety attached to its Ser/Thr-rich domain [15]. Once thrombin, the pivotal protein in the coagulation cascade, binds to TM on the surface of the EC layer, thrombin’s fibrinogen-cleaving activity is inhibited. In addition, this binding will also alter thrombin from a procoagulant to an anticoagulant by activating protein C (PC) to initiate the PC anticoagulation pathway [15].
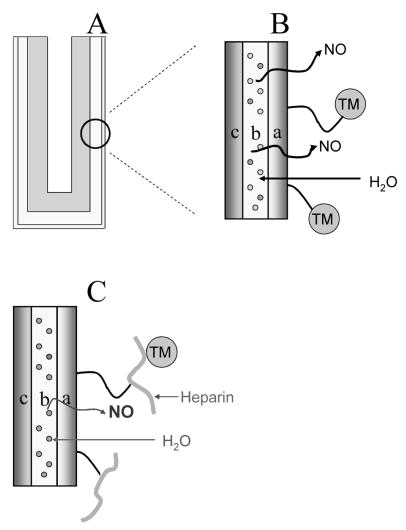
Herein, polymeric coatings with controlled NO release and surface-bound active TM alone or along with heparin are prepared and characterized (Fig. 1). Two approaches are examined. In both cases, an underlying NO release polymer film is employed in which a lipophilic diazeniumdiolated NO donor molecule is incorporated (Fig. 1A). This layer provides the NO release once water partitions into the film. In the first scheme in which TM is immobilized alone, an outer polymer coating (over the inner NO release film) is modified with free carboxyl groups (see Fig. 1B) so that TM can be covalently linked via amide bonds. In the second approach, heparin is first immobilized to an outer layer possessing free amine groups, and then TM is coupled to heparin using the excess carboxyl groups on heparin to create amide bond with amine groups on TM (see Fig. 1C).
Fig. 1.
A) Schematic of SR catheter sleeve (made with SR tube (OD = 1.19 mm, ID = 0.64 mm, L = 1.5 cm) sealed at one end with RTV 3140) with biomimetic bilayer coating. B) Illustration of this bilayer polymeric coating with combined NO release and surface-bound TM: a) top layer: carboxylated CarboSil with surface-bound TM; b) underlying layer: PurSil matrix doped with DBHD/N2O2 (structure shown in Fig. 2) and KTpClPB; c) SR substrate. C) Illustration of this bilayer polymeric coating with combined NO release and surface-bound heparin and TM: a) top layer: aminated CarboSil with TM attached onto the surface-bound heparin; b) underlying layer: PurSil matrix doped with DBHD/N2O2 and KTpClPB; c) SR substrate.
The effect of TM immobilization chemistry on the NO release properties of the underlying film is examined. Further, it is shown that in addition to releasing NO at physiologically relevant fluxes, the resulting polymer coatings possess significant amounts of immobilized TM or TM plus heparin biological activities, as determined by activated PC (APC) and anti-Factor Xa (anti-FXa) chromogenic assays.
2. Experimental Section
2.1 Materials and Instrumentation
Recombinant soluble human TM (TM (CS+), with a chondriotin sulfate moiety attached to TM’s Ser-Thr rich domain) from a high-producing AV12-664 cell line was purified as described previously [16]. Human PC, as well as the chromogenic substrates S-2366 and S-2222, were generous gifts from Instrumentation Laboratory Inc. (Lexington, MA). Human APC, human α-thrombin and human AT III were purchased from Haematologic Technologies Inc. (Essex Junction, VT). Hirudin (from leeches), porcine heparin (171 USP units/mg), glutaraldehyde (25% aqueous solution), Triton X-100, Trizma® Pre-set crystals (for preparing Tris buffer), 2-(N-morpholino)ethanesulfonic acid hydrate (for preparing MES buffer), phosphate buffered saline (PBS, pH 7.4), PBS (containing Tween 20, pH 7.4), bromophenol blue and dibutylamine (DBA) were all purchased from Sigma (St. Louis, MO). Bovine serum albumin (BSA) solution was obtained from Invitrogen (Carlsbad, CA). Potassium tetrakis(4-chlorophenyl) borate (KTpClPB) and triglycine (glycyl-glycyl-glycine, TG) were obtained from Fluka (Ronkonkoma, NY). Glycine (Gly), 0.6 mL Fisherbrand low retention microcentrifuge tubes and 96-well polystyrene (PS) microtiter plates were obtained from Fisher Scientific (Fair Lawn, NJ). Diprimary amine terminated poly(ethylene oxide) (DPA-PEO, with 10 repeating ethylene oxide units) was generously donated by Tomah Inc. (Milton, WI). PurSil 20 80A and CarboSil 20 90A were from The Polymer Technology Group (Berkeley, CA).
Silicone rubber catheter sleeves were made from silicone tubing (0.64 mm ID/1.19 mm OD), purchased from Helix Medical Inc. (Carpinteria, CA). Polypropylene (PP) 96-well microplates with round ‘U’ bottom were purchased from Evergreen Scientific (Los Angeles, CA). Nanosep centrifugal devices (Mw cutoff = 5K) were purchased from Pall Life Sciences (Ann Arbor, MI). Whatman 13 mm ZC syringe filters were obtained from Whatman (Florham Park, NJ). Hexamethylene diisocyanate (HMDI, Aldrich (St. Louis, MO)) was distilled at reduced pressure right before use. The 3140 RTV SR was purchased from Dow Corning Corporation (Midland, MI). Pyridine (Aldrich) was dried and distilled over 4M molecular sieves right before the reaction. Dibutyltin dilaurate (DBTDL), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS) and p-toluenesulfonic acid monohydrate (TsOH) were all used as received from Aldrich. Citrate/phosphate/dextrose solution was purchased from Hospira Inc. (Lake Forest, IL). Lactate dehydrogenase (LDH) assay kits were purchased from Roche Applied Sciences (Indianapolis, IN). Oregon Green 488 (OG488) succinimidyl ester was purchased from Molecular Probes, Inc. (Eugene, OR). DBHD/N2O2 was synthesized by treating N,N′-dibutyl-1,6-hexanediamine (DBHD, Aldrich) with NO gas (80 psi, Cryogenic Gases (Detroit, MI)) at room temperature for 24 h as previously described [11].
FTIR spectra were collected on a Perkin-Elmer BX FT-IR system (Wellesley, MA). Nitric oxide flux from polymer coatings was measured by a chemiluminescent NO analyzer (NOA™ 280, Sievers Instruments, Inc. (Boulder, CO)). A scanning electron microscope (Philips XL30 FEG, FEI Company (Hillsboro, OR)) was used to characterize the morphology of the coatings. Static air–water contact angles were measured by using a Cam-100 Optical Contact Angle Goniometer (KSV Instruments Ltd. (Monroe, CT)). Fluorescence spectra were recorded with a Shimadzu RF-1501 spectrofluorophotometer (Tokyo, Japan). The UV-Vis absorbance changes in the chromogenic assays were measured with a microtiter plate reader (UV-IR-Vis Multiskan Spectrum Model 349, MTX Lab Systems, Inc. (Vienna, VA)).
2.2. Synthesis of Carboxylated and Aminated CarboSil
The urethane groups on CarboSil backbone were used to couple with HMDI through an allophanate reaction in the presence of a tin catalyst (DBTDL) as reported previously [13]. The resulting polymer with pendant isocyanate groups (CarboSil-NCO) was further used to prepare carboxylated and aminated CarboSil.
The carboxylation of CarboSil-NCO was carried out via a modified urea-forming reaction [17, 18]. Equimolar of pyridine was first mixed with a glycine or triglycine suspension in anhydrous dimethylacetamide (DMAc, 10 w/v%) overnight at 60 °C. CarboSil–NCO was dissolved in DMAc to make a 10 w/v% solution and mixed with an excess of freshly prepared pyridinyl glycine/triglycine suspension. The reaction mixture was stirred under argon at 60 °C for 2 d, and then precipitated in copious ethanol/H2O (4:1, v/v). The precipitate was filtered and washed with ethanol/H2O (4:1, v/v) twice and then soxhlet extracted with methanol. Afterwards, it was washed again with ethyl ether and dried under vacuum for 2 d and then stored at −20 °C.
The amination of CarboSil-NCO was carried out according to a previously reported method [13]. Figure 2 summarizes the general carboxylation and amination procedures employed to modify CarboSil.
Fig. 2.
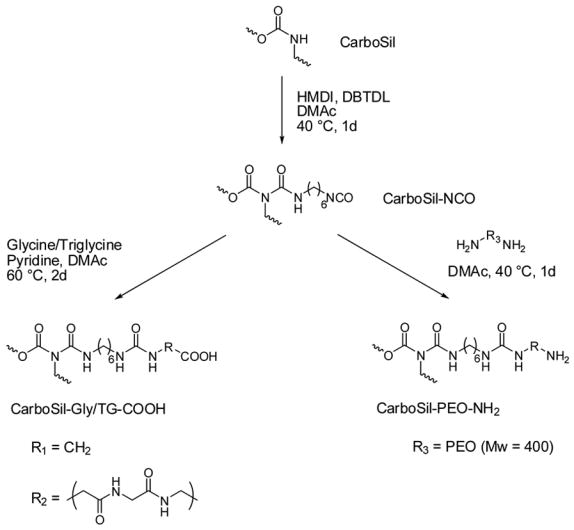
Synthesis of carboxylated and aminated CarboSil.
2.3. Determination of Isocyanate Group Concentration on CarboSil-NCO and Amino Group Concentration on CarboSil-PEO-NH2
The concentration of pendant isocyanate groups on CarboSil-NCO was determined via a modified titration method [19]. Approximately 20 mg CarboSil-NCO was dissolved in 20 mL DMAc under argon to form a homogeneous solution. An excess amount of DBA was added and the mixture was shaken under argon at room temperature overnight. The remaining base was titrated with an isopropanol solution of TsOH, and the endpoint was indicated by bromophenol blue, whose color changed from purplish blue to light yellow.
A similar method was adopted for the determination of the amine content of CarboSil-PEO-NH2. Approximately 20 mg CarboSil-PEO-NH2 was dissolved in 20 mL DMAc. The amino groups were titrated with TsOH solution, with bromophenol blue as the indicator.
2.4. Preparation of Polymeric Coatings with Underlying NO Release Layer and Surface Immobilized Heparin and Thrombomodulin
The underlying NO release polymeric coatings were prepared using a method similar to that previously reported by our group [13]. Then, a carboxylated or aminated CarboSil top layer was applied by dip-coating twice in a CarboSil-Gly/TG-COOH or CarboSil–PEO–NH2 solution in DMAc/THF. The polymer concentrations ranged from 1 to 4 w/v% to achieve outer layers with different thicknesses. The catheter sleeves were allowed to dry overnight, followed by vacuum drying at room temperature for additional 2 d to remove any residual solvent. Figure 1 illustrates the final bilayer polymeric coating configurations on SR catheter sleeves. Porcine heparin (unfractionated) was covalently immobilized onto the aminated CarboSil surface as previously reported [13].
To immobilize TM, the carboxyl groups of CarboSil-Gly/TG-COOH and CarboSil-PEO-Hep surfaces were activated by 100 mM EDC and 50 mM NHS in PBS (pH 7.4) at room temperature. The activated carboxyl groups were then reacted with the amine groups on the lysine residues of TM (10 μg/mL in PBS, pH 7.4) at 4 °C overnight [20]. The polymeric coating was then extensively washed with PBS (pH 7.4, containing Tween 20) and then DI water to rinse off any noncovalently-linked TM. The dual-acting polymeric coatings, with NO donor within the underlying PurSil layer and TM bound onto its top layer, were then further characterized (see below).
2.5. Surface Characterization of Multifunctional Coatings
Static air–water contact angles were determined by a sessile drop method using a Cam-100 Optical Contact Angle Goniometer at ambient humidity and temperature. Polymer coated surfaces for contact angle measurements were prepared via spin coating a polymer solution (0.1 w/v% in THF, filtered with Whatman 0.45 μm PTFE syringe filter) onto glass slides at 600 rpm for 40 s. The polymer coated glass slides were further treated to immobilize TM and/or heparin as described above. All the sample slides were first dried in nitrogen overnight and then under vacuum for another 2 d. For each polymer surface, 20 separate drops were examined on two different sample slides to obtain the average contact angle values reported.
SEM was performed with a Philips/FEI XL30 FEG SEM Electron Microscope. The electron beam was operated at 15 kV and the sample chamber pressure was 10−6 torr. Polymeric coatings were dried under vacuum for 2 d and then sputtered with gold prior to acquiring the SEM images.
2.6. Estimation of the Amount of Immobilized hTM
Thrombomodulin was labelled with OG488 succinimidyl ester as follows. To a solution of 150 μL of carbonate-bicarbonate buffer (pH 9.0), 50 μL of OG488 solution (10 mg/mL in DMSO) was added, followed by 150 μL aqueous TM solution (0.8 mg/ml). The mixture was gently vortexed at room temperature in the dark for 2 h. After the coupling reaction, the unreacted OG488 was removed by dialysis using centrifuge devices with a cutoff molecular weight of 5K.
TM-OG488 was immobilized onto CarboSil-Gly surfaces in a similar manner as non-labelled TM. After immobilization, the surface-bound TM-OG488 was hydrolyzed with 2 N NaOH for 1 h at 121°C using an autoclave. After cooling the hydrolyzed mixture to room temperature, acetic acid was added, and the fluorescence intensity of the mixture was measured using 495 nm for excitation and 520 nm for emission. Blank CarboSil-Gly polymeric coatings and solutions with known TM-OG488 concentrations were also autoclaved under the same condition to be used as controls and standards.
2.7. Activity of Surface-Bound Thrombomodulin and Heparin
The bioactivity of surface-bound TM was analyzed by a chromogenic PC assay [16]. In a 0.6 mL microcentrifuge tube, the 1.5 cm long polymer coated SR catheter sleeve (surface area = 0.572 cm2) with immobilized TM was fully immersed into a solution composed of 80 μL BSA solution (7.5 w/v%), 8 μL thrombin solution (100 μg/mL), and 192 μL 20 mM Tris buffer (pH 7.4, with 3 mM CaCl2, 100 mM NaCl and 0.02 % NaN3). After incubation at 37 °C for 5 min, 40 μL of PCsolution (100 μg/mL) was added and the mixture was incubated at 37 °C for 10 min. Then, 80 μL of hirudin (204.8 unit/mL) was added to bring the total volume to 400 μL and the mixture was further incubated at 37 °C for 5 min. The 400 μL mixture was then evenly divided into 4 wells of a PP 96-well microtiter plate. Ninety μL of Tris buffer (pH 7.4) and 10 μL of 2 mg/mL S-2366 substrate solution were then added and absorbance change at 405 nm was measured immediately by a Labsystems Multiskan RC 96-well microplate reader. Control experiments were also performed for surfaces without immobilized TM (carboxylated, aminated and heparinized polymeric surfaces), using the exact same assay procedure.
The stability of surface-bound TM was also tested both in PBS and sheep plasma using this APC assay. Arterial blood from pregnant ewes, weighing 70 ± 14 kg, was drawn into a 9:1 volume of blood anticoagulant (citrate/phosphate/dextrose) solution. The citrated whole blood was centrifuged at 110 g for 15 min at 22 °C and plasma was collected from the supernatant. To re-establish platelet activity, CaCl2 was added to the plasma to raise [Ca2+] by 2 mM.
The bioactivity of surface immobilized heparin was determined by a chromogenic anti-FXa assay [12]. In a microcentrifuge tube, the 1.5 cm long SR catheter sleeve with heparinized polymer coating (surface area = 0.572 cm2) was fully immersed in a solution composed of 80 μL BSA solution (7.5 w/v%), 80 μL AT III solution (100 μg/mL), and 232 μL PBS. After incubation at 37 °C for 2 min, 8 μL of 20 μg/mL FXa was added and the mixture was further incubated at 37 °C for 1 min. The 400 μL mixture was then evenly divided into 4 wells of a PP 96-well microtiter plate. Ninety μL of PBS and 10 μL of 2 mg/mL S-2222 solution were then added. Absorbance changes at 405 nm were measured immediately by the microplate reader. Experiments were also performed using non-heparinized surfaces (aminated polymer surface) and the heparinized surface after subsequent TM immobilization.
2.8. Chemiluminescence Measurements of NO Release
Nitric oxide released from the polymeric coatings was measured using a Sievers Chemiluminescence NO Analyzer as described in detail within previous work [11, 13, 21].
2.9. In Vitro Platelet Adhesion Experiments Using Sheep Plasma
A lactate dehydrogenase assay was used to quantify platelet adhesion on polymeric coatings with an NO-release underlying layer and CarboSil-Gly top layer prepared in PP 96-well microtiter plates. Thrombomodulin was immobilized using the method described above. Microtiter plates with control polymeric coatings were also prepared. After incubation with sheep plasma, adhesion of platelets was determined by measuring LDH enzymatic activity derived from the platelets via a method reported recently by Wu et al. [22].
SEM images for surfaces of the various polymeric films were also obtained. The polymer coatings were prepared via sequentially spin coating a DBHD/N2O2 containing PurSil cocktail and then a CarboSil-Gly solution in DMAc/THF onto glass slides at 600 rpm for 40 s as the underlying NO release and the top layers, respectively. All the sample slides were first dried in nitrogen overnight and then under vacuum for 2 d. The polymer coated glass slides were then further treated to immobilize TM as described above. After plasma incubation, adhered platelets in some wells were fixed with 4% glutaraldehyde for 1 h and then dehydrated in a series of ethanol solutions and dried overnight. The polymeric coatings with adhered platelets were sputtered with gold prior to acquiring the SEM images.
3. Results and Discussions
3.1. Preparation and Characterization of Outer Carboxylated/Aminated CarboSil Layer
Carboxylation was carried out on CarboSil, a silicone-poly(carbonate)urethane copolymer that is used for biomedical applications [3, 23]. This polymer has been suggested for longer-term in vivo implants, including vascular grafts, owing to the greatly improved biostability and biomcompatibility, compared to the classical polyether type polyurethanes.
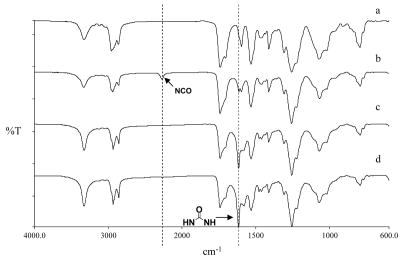
The urethane groups on the polymer backbone were first coupled with a diisocyanate compound (HMDI) [24]. The coupling was confirmed using IR by the appearance of a NCO band at 2266 cm−1, and another new band at 1619 cm−1 (corresponding to urea absorption) after reaction for 1 d (see Figs. 3a and b). The concentration of the isocyanate groups was determined to be 401 ± 3 μmol/g (n = 3) via the modified titration method [19]. Prolonging the reaction time would increase the isocyanate concentration (data not shown) yet the polymer also became more and more difficult to dissolve due to the increasing extent of crosslinking between separate polymer chains.
Fig. 3.
Typical IR spectra of CarboSil and its derivatives: a) CarboSil; b) CarboSil-NCO; c) CarboSil–Gly–COOH; d) CarboSil-TG-COOH.
The resulting polymer with pendant free isocyanate groups was further reacted with the amino groups of glycine/triglycine to form carboxylated CarboSil (CarboSil-Gly/TG-COOH). The disappearance of the 2266 cm−1 peak and increased intensity at 1619 cm−1 in the IR spectra (Figs. 3c and d) verified the formation of the second urea bond at the expense of the isocyanate group.
The preparation of the aminated CarboSil was described previously [13]. The concentration of amine groups on the CarboSil-PEO-NH2 in this study was determined to be 221 ± 4 μmol/g (n = 3) by titration. Compared to that of the isocyanate groups in CarboSil-NCO (401 ± 3 μmol/g), the decrease of functional group concentration may be attributed to the crosslinking of separate polymer chains by the diamine.
3.2 Immobilization of TM on Carboxylated CarboSil as well as Heparin and TM on Aminated CarboSil
A number of methods have been adopted for the immobilization of TM using a variety of surface functional groups, including carboxylic acid [20], amine [25], and trichlorotriazine [26] to anchor TM through the formation of covalent linkages. It has also been reported that TM can be incorporated within a phospholipid membrane via in situ photopolymerization [27]. Here, we chose to introduce carboxylic groups (from glycine, triglycine or heparin) on the outer CarboSil polymeric surface to form stable amide bonds with the amine groups on the TM. The reaction can be carried out in very mild conditions (pH 7.4, room temperature or 4 °C) and this helps to minimize any possible deterioration of TM’s biological activity.
In one approach, heparin was covalently immobilized on the aminated CarboSil surfaces to generate COOH groups for the immobilization of TM. As a highly negatively charged polysaccharide with a molecular weight ranging from 5 kDa to 20 kDa, heparin (unfractionated) has 18 COOH groups per molecule on average [12]. The EDC/NHS/Hep-COOH ratio used corresponds to a value where only one COOH group per every heparin molecule can react with the polymer surface amine groups so that maximal activity of heparin could be maintained [12], and the remaining COOH groups on immobilized heparin could be used to immobilize TM.
3.3. Surface Characterization
SEM images (Fig. 4a, b) show that the surface of the polymeric coatings was smooth on micrometer scale and that no observable change in roughness is observed. Also, there was no discernible interface between the top layer and the underlying layer. The total thickness of these bilayer coatings was ca. 35–50 μm (Fig. 4c).
Fig. 4.
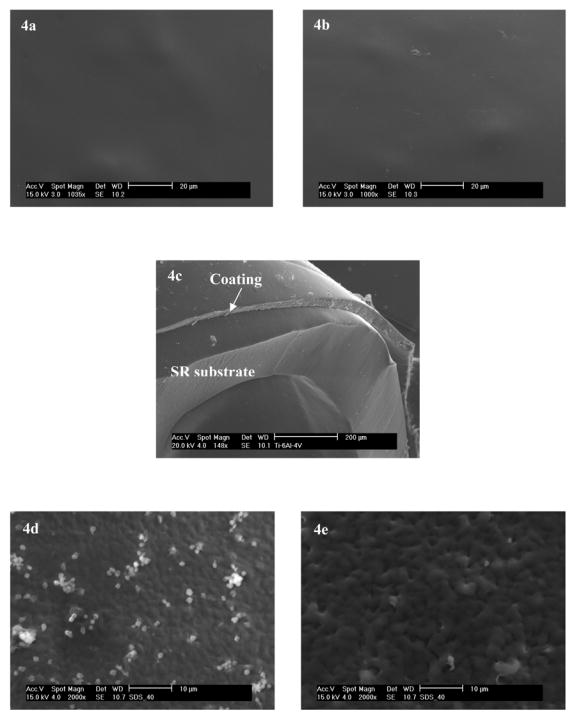
a) and b) are surfaces before and after TM immobilization with top-coatings made by dip-coating in 2 wt% solution of CarboSil-Gly-COOH in DMAc/THF, respectively. The underlying layers of all coatings were doped with 8 wt% of DBHD/N2O2 and an equimolar amount of KTpClPB. c) Typical cross section of the SR catheter sleeves onto which the bilayer polymeric coatingwas applied. The thickness of the bilayer coating was approx. 35–50 μm. d) and e) are SEM micrographs of adhered platelets on polymeric coatings after 1 h incubation in sheep plasma. d) Control polymer with PurSil as the underlying layer and CarboSil-Gly as the top layer; e) Polymeric coating with its underlying layer made of PurSil doped with 8 wt % DBHD/N2O2 and its top layer made of CarboSil-Gly with surface-immobilized TM. The different roughness is due to different coating methods: a), b) and c) were dip-coated while d) and e) were spin-coated.
Chemically modifying the polymer surface also led to significant changes in the air–water contact angle of the material surface. The contact angle of CarboSil decreased from 105 ± 3° to 91 ± 2° after glycine was attached, and that of CarboSil-Gly-TM further decreased to 69 ± 3°, showing that the surface becomes quite hydrophilic after the immobilization of the TM protein.
3.4. Activity of Surface-Bound TM and Heparin
The anti-coagulant activity of the immobilized TM was evaluated by a chromogenic APC assay. Activation of PC by thrombin alone is slow and has no physiological function. However, when thrombin binds to TM in the presence of Ca2+, the rate by which thrombin activates PC will increase by 20,000-fold [15]. The activated PC can be quantified by measuring the cleavage rate of the chromogenic substrate S-2366 (Glu-Pro-Arg-pNA·HCl) [16]. For immobilized TM, its activity is expressed in terms of nanograms of APC generated per square centimeter per min.
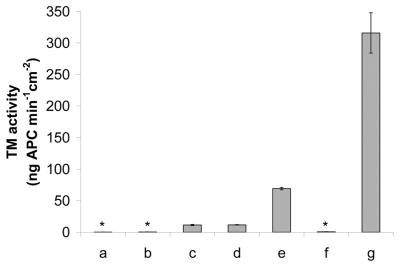
The immobilized TM’s activity on CarboSil-Gly-COOH was determined to be 11.7 ± 0.5 and 11.5 ± 0.5 ng APC·cm2·min−1 in the presence and absence of a surface NO flux of 1.0×10−10 mol·cm−1 ·min−1, respectively. Surfaces without immobilized TM or with only physically adsorbed TM showed negligible activity (0.1 ± 0.2 and 0.3 ± 0.2 ng APC·cm2·min−1, respectively (see Fig. 5)). This confirmed that the anticoagulant activity comes from covalently bound TM and not physically adsorbed TM.
Fig. 5.
TM activity of modified polymeric surfaces (determined by PC assay): a) CarboSil-Gly-COOH in the absence of NO; b) CarboSil-Gly-COOH with physically adsorbed TM in the absence of NO; c) CarboSil-Gly-TM in the absence of NO; d) CarboSil-Gly-TM in the presence of an NO flux of ca. 1.0×10−10 mol·cm−2·min−1; e) CarboSil-TG-TM in the presence of an NO flux of ca. 1.0×10−10 mol·cm−2·min−1. f) CarboSil-PEO-Hep in the presence of an NO flux of ca. 1.0×10−10 mol·cm−2·min−1; g) CarboSil-PEO-Hep-TM in the presence of an NO flux of ca. 1.0×10−10 mol·cm−2·min−1. (n = 4 for each set of data)
The absolute amount of TM immobilized on CarboSil-Gly surfaces was estimated to be 1.77 ± 0.26 pmol/cm2 as determined by a fluorescence method based on labeling TM with OG488. This indicates that approx. 70% of the surface is covered with TM. Such a value should correspond to a much higher PC activating capability than measured by the APC assay (see above). A possible reason for less activity is that the short spacer (i.e., glycine) between TM molecules and the polymeric surface might hinder both the mobility of TM and the accessibility of its substrates. Indeed, with longer spacers such as TG and PEO-Hep as described below, a significant increase of TM biological activity could be detected using the same immobilization chemistry.
In the presence of a surface NO flux of 1.0×10−10 mol·cm−1 ·min−1, the immobilized TM’s activity on CarboSil-TG-COOH was determined to be 69.2 ± 1.9 ng APC·cm2·min−1 (see Fig. 5). The 6-fold increase in the activity of surface-bound TM on CarboSil-TG-COOH compared to CarboSil-Gly-COOH may be attributed to the longer chain that links TM onto the surface and hence better accessibility of thrombin and PC to TM’s active site.
Neither CarboSil-PEO-NH2 or CarboSil-PEO-Hep showed observable response in the PC assay. However, CarboSil-PEO-Hep-TM exhibited a significant TM activity of 316.0 ± 31.9 ng APC·cm2·min−1 (see Fig. 5). This is quite reasonable given that the PEO moiety together with heparin, would form an extremely flexible spacer between TM and the polymeric surface; hence, the immobilized TM should be quite accessible to thrombin and PC. However, it is also possible that the high local concentration of negatively-charged heparin may also contribute to the strong association of thrombin with immobilized TM for PC activation. It is conceivable that an approximation model, akin to those described for serpin-heparin-protease ‘ternary complex’ formation, may also contribute to the apparent activity increase [28].
TM has long been recognized for its thermostability [29]. In this study, we evaluated the stability of the surface-bound TM. An insignificant loss (< 10%) of activity of TM immobilized on CarboSil-Gly-COOH was observed over a five-month period when CarboSil-Gly-TM coatings were stored in PBS at 4 °C (n = 4). Furthermore, almost 70 % of the original activity of TM is maintained over a four-week period in PBS at 37 °C (n = 4). Based on our extensive experience with the NO release coatings of the type used in this work, constant fluxes of NO for up to 2 weeks at 37°C is typically observed [11, 13, 22] which is quite compatible with the TM lifetime under similar conditions.
The polymeric surfaces with immobilized TM were also incubated in sheep plasma for 1 hour at 37°C to assess whether plasma components can decrease TM activity. However, we found that functional TM activity (based on APC assay) actually increased slightly after exposure to the plasma. One possible reason is that some components in plasma might adhere onto the polymeric surfaces and cause the activation of PC and/or the cleavage of the chromogenic substrate. Further experiments are needed to verify this hypothesis.
The activity of surface-bound heparin immobilized by the same procedure used here have been evaluated in our earlier work and found to be 4.80 ± 0.08 mU/cm2 on poly(vinyl chloride) (PVC) and 6.39 ± 0.08 mU/cm2 on PU surfaces [13]. The activity of surface-bound heparin here was found to be 5.34 ± 0.71 mU/cm2, which is comparable to the previous results. After the immobilization of TM, heparin’s activity drops to 4.34 ± 0.20 mU/cm2, a possible result of the loss of COOH groups during the TM immobilization process as well as the steric hindrance from the large TM molecule, now linked to the surface appended heparin.
3.5. NO Release from Polymeric Coatings with Surface-Bound TM and/or Heparin
The release of NO from the underlying polymer film containing the N-diazeniumdiolates (DBHD/N2O2) is driven by protons [11]. Therefore, exposure of this class of NO donors to physiological conditions stimulates NO release. The addition of KTpClPB, a lipophilic tetraphenylborate derivative, into the polymer coating is critical to maintain a low enough pH within the organic polymer phase to promote continued NO release [11]. The flux of NO can range from 0.5 to 60 × 10−10 mol cm−2 min−1 (physiological level ca. 1.0 × 10−10 mol cm−2 min−1 [30]) and the duration can be longer than 2 weeks (data not shown), depending on the specific polymer film composition employed [11,13].
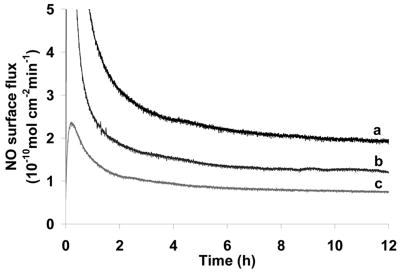
Herein, we also found that by increasing the thickness of the top-layer, the NO release rate decreases. By dip-coating the SR catheter sleeves (pre-coated with NO donor containing layer) in carboxylated/aminated CarboSil solutions of different concentrations (1 to 4 w/v%), top layers with different thickness could be applied which would result in different NO fluxes (see Fig. 6).
Fig. 6.
NO flux levels of DBHD/N2O2-loaded SR catheter sleeves before TM immobilization. The underlying layers of all three coatings were loaded with 8 wt % of DBHD/N2O2 and an equimolar KTpClPB, while the top-coatings were made by dip-coating the catheter sleeves into 1 % (a), 2 % (b) and 4 (c) % (w/v) solutions of CarboSil-Gly-COOH in DMAc/THF, respectively.
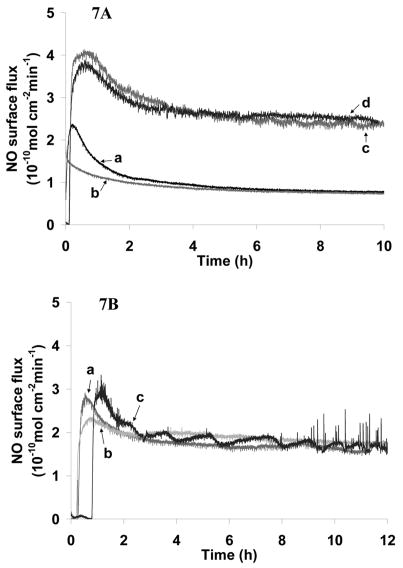
Most importantly, the immobilization procedures, which involved the contact between the coatings and water for ca. 12 h (TM immobilization on carboxylated CarboSil) to 18 h (TM and heparin immobilization on aminated CarboSil), have very limited effect on the NO flux (see Figs. 7A and B). It has been reported previously that ca. 1–2% of NO loss was found for a PVC coating (PVC:DOS = 1:1) after ca. 8–10 h in contact with aqueous solution at room temperature [13]. Thus, it can be predicted that the NO loss during the TM and heparin immobilization process using the more hydrophobic PurSil polymer reported here (with DBHD/N2O2 doped PurSil as the underlying layer and functionalized CarboSil as the top layer) should also be quite small compared to the NO reservoir in the underlying polymer film.
Fig. 7.
A. NO flux profiles of SR catheter sleeves before/after TM immobilization. The underlying layers of all four coatings were loaded with 8 wt % of DBHD/N2O2 and an equimolar amount of KTpClPB. The top-coatings were made by dip-coating the catheter sleeves in 4 % (w/v) solution of CarboSil-Gly-COOH (a and b) or 1 % (w/v) solution of CarboSil-TG-COOH (c and d). Coatings b and d were further immobilized with TM. a) CarboSil-Gly-COOH; b) CarboSil-Gly-TM; c) CarboSil-TG-COOH; d) CarboSil-TG-TM. 7B. NO flux profiles of SR catheter sleeves before and after heparin/TM immobilization. The underlying layers of all three coatings were loaded with 8 wt % of DBHD/N2O2 and an equimolar amount of KTpClPB. The top-coatings were made by dip-coating the catheter sleeves in 1 % (w/v) DMAc/THF solution of CarboSil-PEO-NH2. a) CarboSil-PEO-NH2; b) CarboSil-PEO-Hep; c) CarboSil-PEO-Hep-TM.
3.6. Preliminary In Vitro Study of Blood Compatibility of the Bilayer Polymeric Coatings
In recent years, the lactate dehydrogenase present within platelets has been reported to provide a useful approach to study in vitro platelet adhesion on chemically and/or physically modified surfaces. In previous work, the LDH assay has been used to assess the platelet adhesion on NO-releasing PVC coatings [22]. In this work, a decreased amount (19.8 ± 2.7 %) of adhered platelets was detected on NO-releasing polymeric coatings compared with polymer coatings without NO release. However, the presence of surface-immobilized TM had no significant effect on platelet adhesion. This is expected since NO, being a potent anti-platelet agent, can prohibit platelet activation and adhesion, while TM is an anticoagulant agent which asserts its antithrombotic activity in triggering the PC pathway which will down regulate the coagulation cascade.
Similar results were also observed using SEM to image the platelet adhesion and activation on the various surfaces (see Fig 4d and e as examples). From the SEM images, it can be seen that almost no activation of adhered platelets, not only on polymers with NO release and immobilized TM, but also on control polymeric coatings. This might be due to the already improved biocompatibility of the CarboSil-based top layers, compared to PVC and other less biocompatible polymeric materials.
4. Conclusions
Multifunctional bilayer polymeric coatings have been fabricated with both controlled NO release and surface-bound active TM or combined TM and heparin. The outer layer was made of a commercially available SR-PU copolymer (CarboSil). This outer coating material can be either carboxylated or aminated to enable covalent attachment of TM or heparin and TM. The anticoagulant activity of TM and heparin were evaluated by PC and anti-FXa assay, respectively. It should be noted that the length of the spacer was critical to the activity of surface-bound TM in that longer spacers likely rendered TM’s active site more accessible to its substrates, and this, in turn, resulted in higher activity. Surface-bound TM showed very good stability when stored in PBS both at 4 °C and at 37 °C. Immobilized heparin maintained most of its activity after the immobilization of TM to its COOH functional groups. Further, the immobilization of TM and heparin had little influence on NO release levels, and NO release did not influence the activity of surface-bound heparin and TM.
The combination of controlled NO release as well as immobilized active TM and/or heparin from/on the same polymeric surface suggests that such a synergistic system is potentially applicable towards the development of a truly blood-compatible surface for biomedical devices. More detailed in vivo studies will soon be carried out to assess the true anti-platelet and anticoagulant activity of these new coatings under physiological conditions.
Acknowledgments
We gratefully acknowledge the NIH (EB-00783 and HD-015434) for supporting this work. Special thanks to Instrumentation Laboratory Inc. for providing the PC and chromogenic substrates. We also thank Prof. Zhan Chen, Prof. Raoul Kopelman and Ms. Wei Tang in the University of Michigan Chemistry Department as well as Dr. Kai Sun and Dr. Haiping Sun in the University of Michigan Electron Microbeam Analysis Laboratory for their assistance in surface characterization. We also wish to thank Nathan Gray Lafayette and Candice Marie Hall in the Extracorporeal Membrane Oxygenation Lab at University of Michigan Medical School for preparing the sheep blood used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263(5154):1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 2.Ratner BD. Blood compatibility - a perspective. J Biomater Sci-Polym E. 2000;11(11):1107–1119. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- 3.Xue L, Greisler HP. Biomaterials in the development and future of vascular grafts. J Vasc Surg. 2003;37(2):472–480. doi: 10.1067/mva.2003.88. [DOI] [PubMed] [Google Scholar]
- 4.Moncada S, Palmer RMJ, Higgs EA. Nitric-oxide - physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–142. [PubMed] [Google Scholar]
- 5.Vane JR, Botting RM. Pharmacodynamic profile of prostacyclin. Am J Cardiol. 1995;75(3):A3–A10. doi: 10.1016/s0002-9149(99)80377-4. [DOI] [PubMed] [Google Scholar]
- 6.Woodhouse KA, Weitz JI, Brash JL. Lysis of surface-localized fibrin clots by adsorbed plasminogen in the presence of tissue plasminogen activator. Biomaterials. 1996;17(1):75–77. doi: 10.1016/0142-9612(96)80759-9. [DOI] [PubMed] [Google Scholar]
- 7.Hoylaerts M, Owen WG, Collen D. Involvement of heparin chain-length in the heparin-catalyzed inhibition of thrombin by antithrombin-III. J Biol Chem. 1984;259(9):5670–5677. [PubMed] [Google Scholar]
- 8.Whitelock JM, Iozzo RV. Heparan sulfate: A complex polymer charged with biological activity. Chem Rev. 2005;105(7):2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 9.Esmon CT. The roles of protein-C and thrombomodulin in the regulation of blood-coagulation. J Biol Chem. 1989;264(9):4743–4746. [PubMed] [Google Scholar]
- 10.Munoz EM, Linhardt RJ. Heparin-binding domains in vascular biology. Arterioscl Throm Vas. 2004;24(9):1549–1557. doi: 10.1161/01.ATV.0000137189.22999.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Batchelor MM, Reoma SL, Fleser PS, Nuthakki VK, Callahan RE, Shanley CJ, et al. More lipophilic dialkyldiamine-based diazeniumdiolates: Synthesis, characterization, and application in preparing thromboresistant nitric oxide release polymeric coatings. J Med Chem. 2003;46(24):5153–5161. doi: 10.1021/jm030286t. [DOI] [PubMed] [Google Scholar]
- 12.Wissink MJB, Beernink R, Pieper JS, Poot AA, Engbers GHM, Beugeling T, et al. Immobilization of heparin to EDC/NHS-crosslinked collagen. Characterization and in vitro evaluation. Biomaterials. 2001;22(2):151–163. doi: 10.1016/s0142-9612(00)00164-2. [DOI] [PubMed] [Google Scholar]
- 13.Zhou ZR, Meyerhoff ME. Preparation and characterization of polymeric coatings with combined nitric oxide release and immobilized active heparin. Biomaterials. 2005;26(33):6506–6517. doi: 10.1016/j.biomaterials.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 14.Esmon NL, Owen WG, Esmon CT. Isolation of a membrane-bound cofactor for thrombin-catalyzed activation of protein-C. J Biol Chem. 1982;257(2):859–864. [PubMed] [Google Scholar]
- 15.Chromogenix. Protein C Product Monograph; 1995.
- 16.Parkinson JF, Grinnell BW, Moore RE, Hoskins J, Vlahos CJ, Bang NU. Stable Expression of a Secretable Deletion Mutant of Recombinant Human Thrombomodulin in Mammalian-Cells. J Biol Chem. 1990;265(21):12602–12610. [PubMed] [Google Scholar]
- 17.Moreau JJE, Vellutini L, Man MWC, Bied C, Dieudonne P, Bantignies JL, et al. Lamellar bridged silsesquioxanes: Self-assembly through a combination of hydrogen bonding and hydrophobic-interactions. Chem-Eur J. 2005;11(5):1527–1537. doi: 10.1002/chem.200401012. [DOI] [PubMed] [Google Scholar]
- 18.Pittelkow M, Christensen JB, Meijer EW. Guest-host chemistry with dendrimers: Stable polymer assemblies by rational design. J Polym Sci A1. 2004;42(15):3792–3799. [Google Scholar]
- 19.Bello D, Streicher RP, Woskie SR. Evaluation of the NIOSH draft method 5525 for determination of the total reactive isocyanate group (TRIG) for aliphatic isocyanates in autobody repair shops. J Environ Monitor. 2002;4(3):351–360. doi: 10.1039/b110613a. [DOI] [PubMed] [Google Scholar]
- 20.Sperling C, Salchert K, Streller U, Werner C. Covalently immobilized thrombomodulin inhibits coagulation and complement activation of artificial surfaces in vitro. Biomaterials. 2004;25(21):5101–5113. doi: 10.1016/j.biomaterials.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 21.Oh BK, Meyerhoff ME. Spontaneous catalytic generation of nitric oxide from S-nitrosothiols at the surface of polymer films doped with lipophilic copper(II) complex. J Am Chem Soc. 2003;125(32):9552–9553. doi: 10.1021/ja035775x. [DOI] [PubMed] [Google Scholar]
- 22.Wu YD, Zhou ZR, Meyerhoff ME. In vitro platelet adhesion on polymeric surfaces with varying fluxes of continuous nitic oxide release. J BIOMED MATER RES A. 2007;81A(4):956–963. doi: 10.1002/jbm.a.31105. [DOI] [PubMed] [Google Scholar]
- 23.Gunatillake PA, Martin DJ, Meijs GF, McCarthy SJ, Adhikari R. Designing biostable polyurethane elastomers for biomedical implants. Aust J Chem. 2003;56(6):545–557. [Google Scholar]
- 24.Park KD, Piao AZ, Jacobs H, Okano T, Kim SW. Synthesis and characterization of SPUU-PEO-heparin graft-copolymers. J Polym Sci A1. 1991;29(12):1725–1737. [Google Scholar]
- 25.Akashi M, Maruyama I, Fukudome N, Yashima E. Immobilization of human thrombomodulin on glass-beads and its anticoagulant activity. Bioconjugate Chem. 1992;3(5):363–365. doi: 10.1021/bc00017a002. [DOI] [PubMed] [Google Scholar]
- 26.Han HS, Yang SL, Yeh HY, Lin JC, Wu HL, Shi GY. Studies of a novel human thrombomodulin immobilized substrate: surface characterization and anticoagulation activity evaluation. J Biomater Sci-Polym E. 2001;12(10):1075–1089. doi: 10.1163/15685620152691869. [DOI] [PubMed] [Google Scholar]
- 27.Tseng PY, Jordan SW, Sun XL, Chaikof EL. Catalytic efficiency of a thrombomodulin-functionalized membrane-mimetic film in a flow model. Biomaterials. 2006;27(13):2768–2775. doi: 10.1016/j.biomaterials.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 28.De Cristofaro R, De Candia E, Landolfi R. Effect of high- and low-molecular-weight heparins on thrombin-thrombomodulin interaction and protein C activation. Circulation. 1998;98(13):1297–1301. doi: 10.1161/01.cir.98.13.1297. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki K, Kusumoto H, Hashimoto S. Isolation and characterization of thrombomodulin from bovine lung. Biochim Biophys Acta. 1986;882(3):343–352. doi: 10.1016/0304-4165(86)90257-6. [DOI] [PubMed] [Google Scholar]
- 30.Feelisch M, Stamler JS. Methods in Nitric Oxide Research. West Sussex: John Willey and Sons Ltd; 1996. [Google Scholar]