Abstract
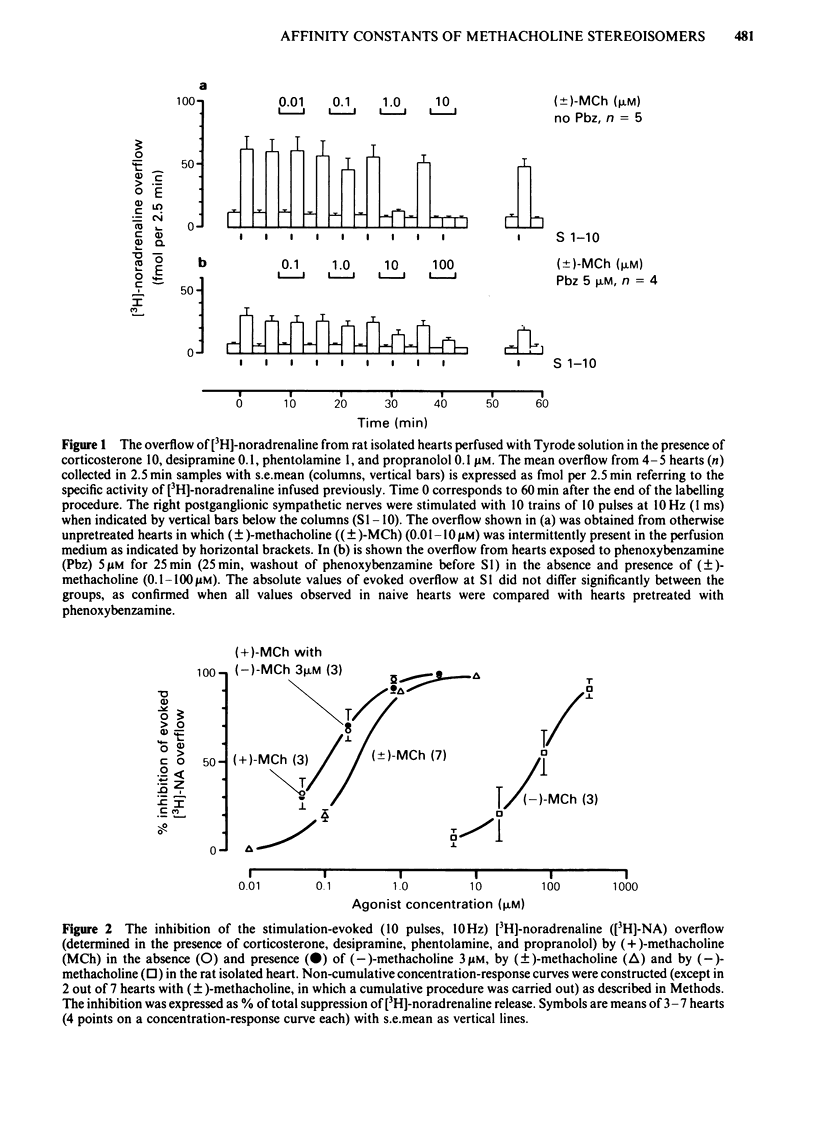
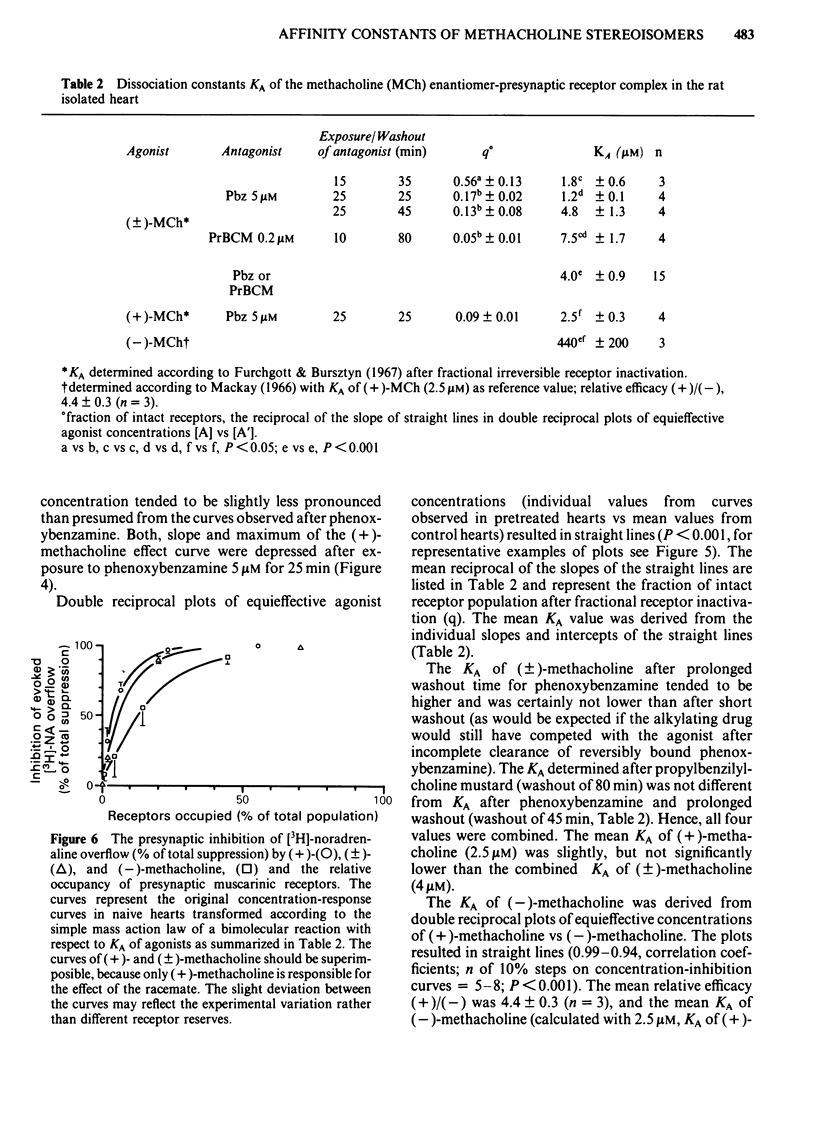
The right postganglionic sympathetic nerves of rat isolated perfused hearts (previously loaded with [3H]-noradrenaline) were stimulated electrically with 10 trains of 10 pulses at 10 Hz. The inhibition by methacholine of stimulation-evoked [3H]-noradrenaline overflow into the perfusate (determined in the presence of corticosterone, desipramine, phentolamine, and propranolol) was taken as a measure for activation of presynaptic muscarinic receptors. The evoked [3H]-noradrenaline overflow was inhibited by (+)-, racemic, and (-)-methacholine in a reversible and concentration-dependent manner. The concentration causing 50% inhibition (IC50) was 0.1, 0.26, and 65 microM, respectively, resulting in an isomeric potency ratio IC50 (+)/IC50(-) of 650. The dissociation constant KA of the (+/-)- or (+)-methacholine-presynaptic receptor complex was determined after fractional receptor inactivation according to Furchgott & Bursztyn (1967) with phenoxybenzamine or propylbenzilylcholine mustard as irreversible antagonists of muscarinic receptors. KA for (-)-methacholine was estimated according to Mackay (1966). KA of (+)-, (+/-)-, and (-)-methacholine were 2.5, 4 and 440 microM, resulting in an isomeric affinity ratio KA (+)/KA(-) of 180. The discrepancy between the isomeric IC50 ratio and the isomeric KA ratio is explained by a higher intrinsic efficacy of the (+)-enantiomer compared to the (-)-enantiomer. Thus, (+)-methacholine has to occupy fewer receptors to induce a given inhibition of release than its antipode as revealed by a plot of fractional receptor occupancy vs response. The results show that, in the effector system of presynaptic muscarinic inhibition, methacholine enantiomers differ greatly not only in affinity for the receptor, but also to some extent in the efficiency of signal transmission, and both parameters contribute to the high isomeric potency ratio. The activity of the racemate is fully accounted for by the activity of the (+)-enantiomer.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronstam R. S., Triggle D. J., Eldefrawi M. E. Structural and stereochemical requirements for muscarinic receptor binding. Mol Pharmacol. 1979 Mar;15(2):227–234. [PubMed] [Google Scholar]
- BECKETT A. H., HARPER N. J., CLITHEROW J. W. The importance of stereoisomerism in muscarinic activity. J Pharm Pharmacol. 1963 Jun;15:362–371. doi: 10.1111/j.2042-7158.1963.tb12799.x. [DOI] [PubMed] [Google Scholar]
- Besse J. C., Furchgott R. F. Dissociation constants and relative efficacies of agonists acting on alpha adrenergic receptors in rabbit aorta. J Pharmacol Exp Ther. 1976 Apr;197(1):66–78. [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- Birdsall N. J., Hulme E. C., Burgen A. The character of the muscarinic receptors in different regions of the rat brain. Proc R Soc Lond B Biol Sci. 1980 Feb 13;207(1166):1–12. doi: 10.1098/rspb.1980.0011. [DOI] [PubMed] [Google Scholar]
- Black J. W., Leff P. Operational models of pharmacological agonism. Proc R Soc Lond B Biol Sci. 1983 Dec 22;220(1219):141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Triggle D. J. Stereoselectivity of cholinergic activity in a series of 1,3-dioxolanes. J Med Chem. 1973 Jun;16(6):718–720. doi: 10.1021/jm00264a037. [DOI] [PubMed] [Google Scholar]
- Ehlert F. J., Dumont Y., Roeske W. R., Yamamura H. I. Muscarinic receptor binding in rat brain using the agonist, [3H]cis methyldioxolane. Life Sci. 1980 Mar 24;26(12):961–967. doi: 10.1016/0024-3205(80)90117-4. [DOI] [PubMed] [Google Scholar]
- El-Fakahany E., Richelson E. Phenoxybenzamine and dibenamine interactions with calcium channel effectors of the muscarinic receptor. Mol Pharmacol. 1981 Nov;20(3):519–525. [PubMed] [Google Scholar]
- Ellenbroek B. W., Nivard R. J., van Rossum J. M., Ariëns E. J. Absolute configuration and parasympathetic action: pharmacodynamics of enantiomorphic and diastereoisomeric esters of beta-methylcholine. J Pharm Pharmacol. 1965 Jul;17(7):393–404. doi: 10.1111/j.2042-7158.1965.tb07694.x. [DOI] [PubMed] [Google Scholar]
- Fuder H., Muscholl E., Spemann R. The determination of presynaptic pA2 values of yohimbine and phentolamine on the perfused rat heart under conditions of negligible autoinhibition. Br J Pharmacol. 1983 May;79(1):109–119. doi: 10.1111/j.1476-5381.1983.tb10502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuder H., Rink D., Muscholl E. Sympathetic Nerve Stimulation on the perfused rat heart. Affinities of N-methylatropine and pirenzepine at pre- and postsynaptic muscarine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):210–219. doi: 10.1007/BF00500482. [DOI] [PubMed] [Google Scholar]
- Fuder H. The affinity of pirenzepine and other antimuscarinic compounds for pre- and postsynaptic muscarine receptors of the isolated rabbit and rat heart. Scand J Gastroenterol Suppl. 1982;72:79–85. [PubMed] [Google Scholar]
- Graffe K. H., Stefano F. J., Langer S. Z. Preferential metabolism of (-) 3 H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol. 1973 May 15;22(10):1147–1160. doi: 10.1016/0006-2952(73)90231-1. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Burgen A. S., Mehta P. The binding of antagonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):737–750. [PubMed] [Google Scholar]
- Ikeda S. R., Aronstam R. S., Eldefrawi M. E. Nature of regional and chemically-induced differences in the binding properties of muscarinic acetylcholine receptors from rat brain. Neuropharmacology. 1980 Jun;19(6):575–585. doi: 10.1016/0028-3908(80)90029-5. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience. 1980;5(7):1331–1340. doi: 10.1016/0306-4522(80)90205-5. [DOI] [PubMed] [Google Scholar]
- Mackay D. A general analysis of the receptor-drug interaction. Br J Pharmacol Chemother. 1966 Jan;26(1):9–16. doi: 10.1111/j.1476-5381.1966.tb01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscholl E., Muth A. The effect of physostigmine on the vagally induced muscarinic inhibition of noradrenaline release from the isolated perfused rabbit atria. Naunyn Schmiedebergs Arch Pharmacol. 1982 Aug;320(2):160–169. doi: 10.1007/BF00506316. [DOI] [PubMed] [Google Scholar]
- Muscholl E. Peripheral muscarinic control of norepinephrine release in the cardiovascular system. Am J Physiol. 1980 Dec;239(6):H713–H720. doi: 10.1152/ajpheart.1980.239.6.H713. [DOI] [PubMed] [Google Scholar]
- Parker R. B. Measurement of drug-receptor dissociation constants of muscarinic agonists on intestinal smooth muscle. J Pharmacol Exp Ther. 1972 Jan;180(1):62–70. [PubMed] [Google Scholar]
- Ringdahl B. Determination of dissociation constants and relative efficacies of oxotremorine analogs at muscarinic receptors in the guinea-pig ileum by pharmacological procedures. J Pharmacol Exp Ther. 1984 Apr;229(1):199–206. [PubMed] [Google Scholar]
- Ruffolo R. R., Jr Review important concepts of receptor theory. J Auton Pharmacol. 1982 Dec;2(4):277–295. doi: 10.1111/j.1474-8673.1982.tb00520.x. [DOI] [PubMed] [Google Scholar]
- Ruffolo R. R., Jr, Rosing E. L., Waddell J. E. Receptor interactions of imidazolines. I. Affinity and efficacy for alpha adrenergic receptors in rat aorta. J Pharmacol Exp Ther. 1979 Jun;209(3):429–436. [PubMed] [Google Scholar]
- Ward D., Young J. M. Ligand binding to muscarinic receptors in intact longitudinal muscle strips from guinea-pig intestine. Br J Pharmacol. 1977 Oct;61(2):189–197. doi: 10.1111/j.1476-5381.1977.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud D. R. On the measurement of the affinity of partial agonists for receptors. J Pharmacol Exp Ther. 1969 Nov;170(1):117–122. [PubMed] [Google Scholar]
- Young J. M., Hiley R., Burgen A. S. Homologues of benzilylcholine mustard. J Pharm Pharmacol. 1972 Dec;24(12):950–954. doi: 10.1111/j.2042-7158.1972.tb08925.x. [DOI] [PubMed] [Google Scholar]


