Abstract
MDCKII-MDR1 cell line has been extensively selected as a model to study P-gp-mediated drug efflux. Recently, investigators have employed this cell line for studying influx of peptide prodrug derivatives of parent compounds which are P-gp substrates. Therefore, the objective of this study is to functionally characterize the peptide mediated uptake and transport of [3H] Glycylsarcosine ([3H] Gly-Sar), a model peptide substrate across MDCKII-MDR1 cells. [3H] Gly-Sar uptake from apical (AP) and basolateral (BL) membranes was found to be time dependent and saturable. Michaelis-Menten (Km) constants of [3H] Gly-Sar uptake across the AP and BL directions in MDCKII-MDR1 cell line were found to be 457 ± 37 μM and 464 ± 85 μM respectively. Vmax values in AP and BL directions for the peptide transporters in MDCKII-MDR1 cell line were calculated to be 0.035 ± 0.001 and 0.35 ± 0.034 pmol/min/mg protein respectively. Uptake of [3H] Gly-Sar was significantly inhibited in the presence of aminocephalosporins and ACE-Inhibitors, known substrates for peptide transporters in both the AP and BL directions. Permeability of [3H] Gly-Sar in the BL direction was maximal at pH 4 as compared to pH 5, 6 and 7.4 whereas such permeability in the AP direction was optimal at pH 7.4. Transepithelial transport of [3H] Gly-Sar in the AP-BL direction was significantly lower than from BL-AP direction at all observed pHs. No statistical difference was observed in the transepithelial permeability of [3H] Gly-Sar across both AP and BL directions over 4–10 days of growth period. The present study indicates that peptide transporters are effectively involved in the bidirectional transport of Gly-Sar across MDCKII-MDR1 cell line; the BL peptide transporter can transport Gly-Sar at a greater rate as compared to the AP peptide transporter. Results from these studies suggest the application of MDCKII-MDR1 cell line as a rapid effective tool to study peptide mediated influx of compounds that may be substrates for both P-gp and peptide transporters.
Keywords: Madin-Darby Canine Kidney cells, MDCKII-MDR1, MDCKII-WT, Caco-2 cells, peptide transporter, P-glycoprotein (P-gp), Gly-Sar, Apical (AP), Basolateral (BL), uptake, transport, permeability
1. INTRODUCTION
Madin-Darby canine kidney (MDCK) cells are a dog renal epithelia derived cell line. When grown onto Transwell®, MDCK cells differentiate into columnar epithelium and form tight junctions in a shorter time period than Caco-2 cells (3 days vs. 21 days). MDCK cells and Caco-2 cells have been reported to share many common epithelial cell characteristics(Putnam et al., 2002). A good correlation between the permeation of passively absorbed drugs across Caco-2 cells and MDCK cells suggests that MDCK model may be useful in place of Caco-2 cells as a monolayer model for intestinal mucosa (Irvine et al., 1999). In the late 1990s, a MDCKII-MDR1 cell line was generated by transfecting human mdr1 gene into MDCK cells.
MDCKII-MDR1 cell line is derived from the kidney, and therefore its physiological function is excretory rather than absorptive. The properties of the renal AP and BL peptide transporters differ from those in the intestine as has been previously reported (Terada et al., 2000). The BL peptide transporter in Caco-2 cells mediates the efflux of small peptides whereas the BL peptide transporter in MDCK cells mediates cellular uptake of small peptides from the extracellular space. The AP peptide transporters in MDCK are involved in reabsorption of small peptides from renal tubules and the AP peptide transporters in Caco-2 cells are involved in absorption of peptides from the intestinal fluid(Terada et al., 2000).
MDCKII-MDR1 cell line has been extensively employed to conduct P-glycoprotein (P-gp/MDR1) mediated drug efflux studies (Guo et al., 2002; Williams et al., 2003; Keogh and Kunta, 2006; Rodriguez-Proteau et al., 2006). P-gp, a MDR gene product, is an ATP-dependent drug efflux pump initially described in cancer chemotherapy. This 170-kDa transmembrane protein is an ATP-dependent transporter of a wide range of compounds, including anticancer drugs, protease inhibitors, peptides, steroids, calcium channel blockers and antihistamines (Endicott and Ling, 1989; Gottesman and Pastan, 1993; Pal and Mitra 2006). P-gp-mediated efflux reduces the intracellular accumulation of these compounds, thereby diminishing drug efficacy. P-gp is a multidrug resistance protein which actively effluxes drug molecules out of the cell. P-gp is present in the apical membrane of many critical epithelia and endothelia. It is ubiquitously expressed in various tissues such as intestinal mucosa, brain capillary endothelial cells, biliary canaliculus and kidney tubules. Broad substrate specificity of P-gp is a major factor responsible for sub-therapeutic levels of various drugs in blood and tissues (Varma et al., 2003). Recent reports suggest that the presence of this efflux pump on the brush border membrane of intestinal epithelium not only diminishes permeability of various therapeutic agents but also enhances the metabolism of these molecules by effluxing the drug into the intestinal lumen or blood capillaries thereby increasing the drug exposure to cellular as well as luminal enzymes (Lown et al., 1997; Watkins, 1997; Ito et al., 1999). Several strategies have been employed to bypass or inhibit this efflux transporter and increase the oral bioavailability of such therapeutic agents. One such promising strategy is transporter targeted prodrug derivatization. Prodrugs have been designed such that the modified compounds become substrates of nutrient transporters like peptide transporters leading to enhanced absorption of these compounds across various physiological barriers (Rousselle et al., 2000; Rouquayrol et al., 2002; Rice et al., 2003).
We have recently reported from our laboratory that peptide prodrug modification of compounds that are P-gp substrates resulted in partial evasion of P-gp mediated efflux (Jain et al., 2004; Jain et al., 2005). Following this discovery, we have used MDCKII-MDR1 cell line for studying peptide-mediated drug influx of peptide prodrug derivatives of compounds which were originally P-gp substrates. MDCKII-MDR1 cell line was used to simultaneously delineate the interaction of these prodrugs with P-gp and peptide transporters. Using two different cell lines for this purpose would have posed a difficulty in normalizing and comparing results. Also, Caco-2 cells have been reported to express both P-gp and peptide transporters (Tang et al., 2002). But the disadvantage of using Caco-2 cell monolayers is that Caco-2 cells take 21–28 days to reach confluency and for fully functional expression of various proteins like P-gp and peptide transporters. Moreover, MDCKII-MDR1 cell line has been established to be as good a model of the intestinal mucosa as the Caco-2 cell line (Tang et al., 2002).
Therefore, the purpose of this study is to functionally characterize the AP and BL peptide transporters in MDCKII-MDR1 cell line using [3H] Gly-Sar to investigate the suitability of this cell line for transport studies involving peptide substrates. Both the AP and BL peptide transporter mediated influx of [3H] Gly-Sar was studied. [3H] Gly-Sar permeability across the AP and BL peptide transporters in MDCKII-MDR1 cell line was compared with its permeability across MDCK-Wild type (MDCKII-WT) and Caco-2 cell lines.
2. EXPERIMENTAL SECTION
2.1 Materials
[3H] Glycylsarcosine (Gly-Sar) (4.0/1.0 Ci/mmol) was obtained from Moravek Biochemicals (Brea, CA, USA). MDCKII cells, retrovirally transfected with the human MDR1 cDNA (MDCKII-MDR1) and MDCKII-WT cells were a gift from Dr. Piet Borst (Netherlands Cancer Institute, Amsterdam). Caco-2 cell line was purchased from American Type Culture Collection (ATCC, USA). The growth medium Dulbecco’s modified Eagle Medium (DMEM), Calf serum, Fetal Bovine serum and nonessential amino acids were obtained from Gibco (Invitrogen, Grand Island, NY, USA). Penicillin, streptomycin, sodium bicarbonate, unlabeled (cold) Gly-Sar and HEPES were purchased from Sigma Chemical (St. Louis, MO, USA). Culture flasks (75 cm2 growth area), polyester Transwell® inserts (pore size 0.22 μM and 12mm diameter) and twelve well plates were obtained from Costar (Cambridge, MA, USA). Dulbecco’s modified phosphate buffer saline (DPBS) was prepared with 129 mM NaCl, 2.5 mM KCl, 7.4 mM Na2-HPO4, 1.3 mM KH2PO4, 1 mM CaCl2, 0.7 mM MgSO4, 5.3 mM glucose at pH 7.4. DPBS also contained 25 mM HEPES. These chemicals were of analytical grade and obtained from Sigma.
2.2 Methods
2.2.1 Cell culture
MDCKII-MDR1, MDCKII-WT and Caco-2 cells were cultured at 37°C in a humidified atmosphere with 5% CO2. Cell confluence was assessed by light microscopy. Cells were passaged at 80–90% confluence using 0.25% trypsin EDTA and were seeded at a density of 100,000 cells/cm2 on 12-well tissue culture plates or on Transwell® inserts. Cells were maintained in DMEM, supplemented with 10% calf serum, 100 IU/mL penicillin, 100 μg/mL streptomycin,1% non essential amino acid, 3.7 g of sodium bicarbonate and 25 mM HEPES, pH 7.4. MDCK cells were allowed to grow for 6–8 days. Caco-2 cells were grown for 21 days.
2.2.2 Uptake Studies
Uptake studies were conducted with confluent cell monolayers, 6–8 days post seeding. Medium was aspirated and cells were washed thrice with DPBS pH 7.4. For AP-BL and BL-AP uptakes, 12 well plates and 12 well Transwells were used respectively. Uptake was initiated by adding 1 mL of drug solution containing 0.5μCi/ml [3H] Gly-Sar on the AP side for AP uptakes and 1.5 ml on the BL side for BL uptakes (in the presence or absence of competing substrates). Incubation was carried out over a period of 15–30 minutes. After the incubation period, the cell monolayers were rinsed three times with ice-cold solution (200 mM KCl and 2 mM HEPES) to terminate drug uptake. Cells were lysed overnight (using 1mL 0.1% (wt/vol) Triton X-100 in 0.3N sodium hydroxide) at room temperature. Aliquots (500μL from the AP side or 250 μL from the BL side) were withdrawn from each well and transferred to scintillation vials containing 5mL scintillation cocktail. Samples were then analyzed by liquid scintillation spectrophotometry with a Beckman scintillation counter (Model LS-6500, Beckman Instruments, Inc.). Rate of uptake was normalized to the protein content of each well. Amount of protein in the cell lysate was quantified by the method of Bradford utilizing BioRad protein estimation kit (BioRad, Hercules,CA).
2.2.3 Transport Studies
Transport studies were conducted with MDCKII-MDR1, MDCKII-WT or Caco-2 cell monolayers grown on 12 well transwell inserts (diameter 12mm). Medium was aspirated and cell monolayers were washed three times (10 minutes per wash) with DPBS pH 7.4. Volumes of the AP and BL chambers were 0.5 mL and 1.5 mL respectively. Transport experiments were conducted for a period of three hours. Aliquots (100 μL) were withdrawn at predetermined time intervals i.e. 15, 30, 45, 60, 90, 120, 150 and180 minutes from the receiver chamber and replaced with fresh DPBS pH 7.4 to maintain sink conditions. Dilutions were taken into account for the flux calculations. For pH dependent transport studies, the pH in the donor chamber was varied while the pH of the receiving chamber remained at 7.4. All transport experiments were performed at 37°C. The radioactive samples were analyzed as mentioned above for the uptake experiments.
2.2.4 Data Analysis
Linear regression of the amounts transported as a function of time yielded the rate of transport across the cell monolayer (dM/dt). Rate divided by the cross-sectional area available for transport (A) generated the steady state flux as shown in Eq. 1.
| Eq. 1 |
Permeability was calculated by normalizing the steady state flux to the donor concentration (Cd) of the drug according to Eq. 2.
| Eq. 2 |
Uptake data was fitted to Michaelis-Menten Eq. 3:
| Eq. 3 |
Where V is the total rate of uptake, Vmax is the maximum uptake rate for the carrier-mediated process, Km (Michaelis-Menten constant) is the concentration at half-saturation, and C is the substrate concentration. Data were fitted to above equation with a nonlinear least square regression analysis program (Kaleida Graph Version 3.09, Synergy Software, Reading, PA, USA).
2.2.5 Statistical Analysis
All experiments were conducted at least in quadruplicate (n=4) and results are expressed as mean ± SD. Statistical comparison of mean values were performed using one-way analysis of variance (ANOVA) or Student t test (Graph Pad INSTAT, version 3.1). *P < 0.05 was considered to be statistically significant.
3. RESULTS
3.1 Time course of Gly-Sar uptake in MDCKII-MDR1 cells
Time dependent uptake of [3H] Gly-Sar was carried out from AP and BL direction in MDCKII-MDR1 cells. Figure 1 depicts the time course of [3H] Gly-Sar uptake from AP and BL directions respectively. 10 minutes uptake time was considered as control. [3H] Gly-Sar uptake accelerated in a time-dependent manner and was linear (r2= 0.99) up to 60 minutes in both AP and BL directions. Duration of uptake for all the further experiments was kept constant at 15–30 minutes.
Figure 1.
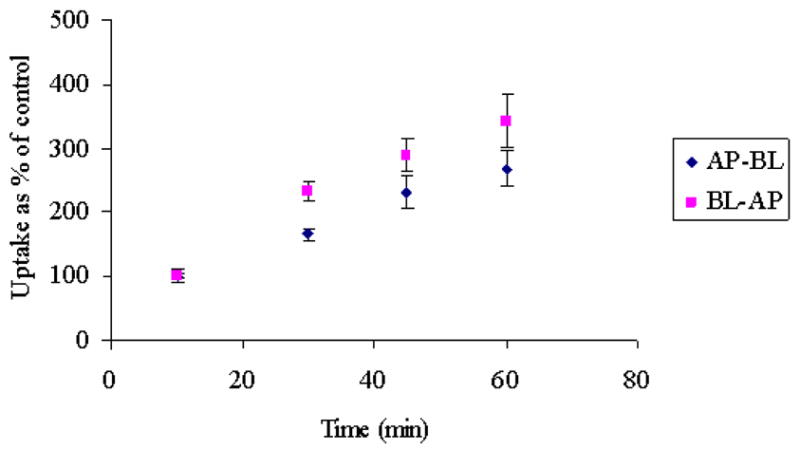
Time dependent AP-BL and BL-AP uptake of [3H] Gly-Sar across MDCKII-MDR1 cells. Each data point represents the mean ± S.D. of 3–4 separate uptake determinations.
3.2 Concentration dependence of Gly-Sar uptake in MDCKII-WT and MDCKII-MDR1 cells
Concentration dependent uptake of [3H] Gly-Sar was carried out to investigate the uptake kinetics of [3H] Gly-Sar from AP and BL directions in MDCKII-WT and MDCKII-MDR1 cells. AP and BL uptakes of [3H] Gly-Sar were saturable with apparent Michaelis-Menten constant (Km) values of 457 ± 37 μM and 464 ± 85 μM respectively in MDCKII-MDR1 cells (Figs. 2 & 3). In MDCKII-WT cells, such AP and BL Km values were 542 ± 37 μM and 106 ± 15 μM in respectively. Vmax values for the AP and BL peptide transporters for MDCKII-MDR1 cell line were calculated as 0.035 ± 0.001 and 0.35 ± 0.034 pmol/mg protein/min respectively and 0.06 ± 0.001 and 0.01 ± 0.022 pmol/mg protein/min respectively for MDCKII-WT cell line.
Figure 2.
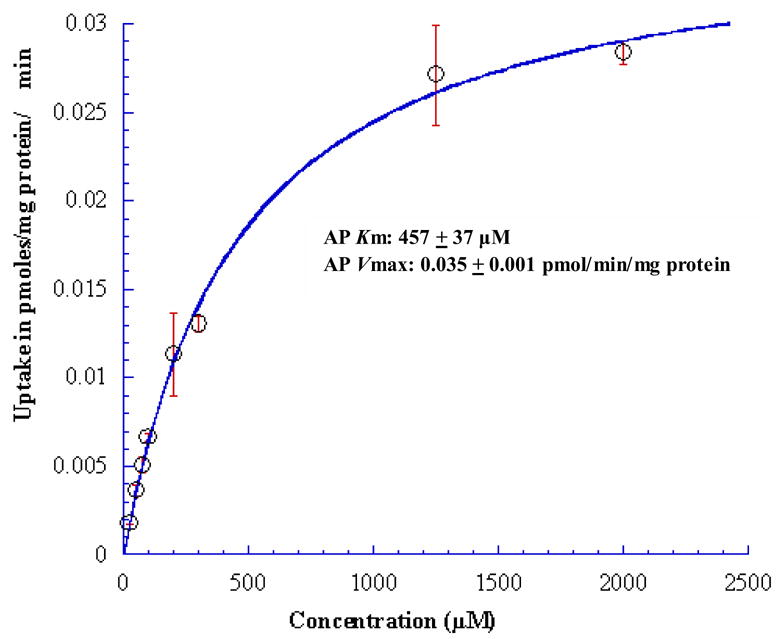
Concentration dependent AP uptake of [3H] Gly-Sar across MDCKII-MDR1 cells. Solid line represents the calculated fit of the data to Michaelis-Menten eq. as described under Materials and Methods. Each data point represents the mean ± S.D. of 3–4 separate uptake determinations
Figure 3.
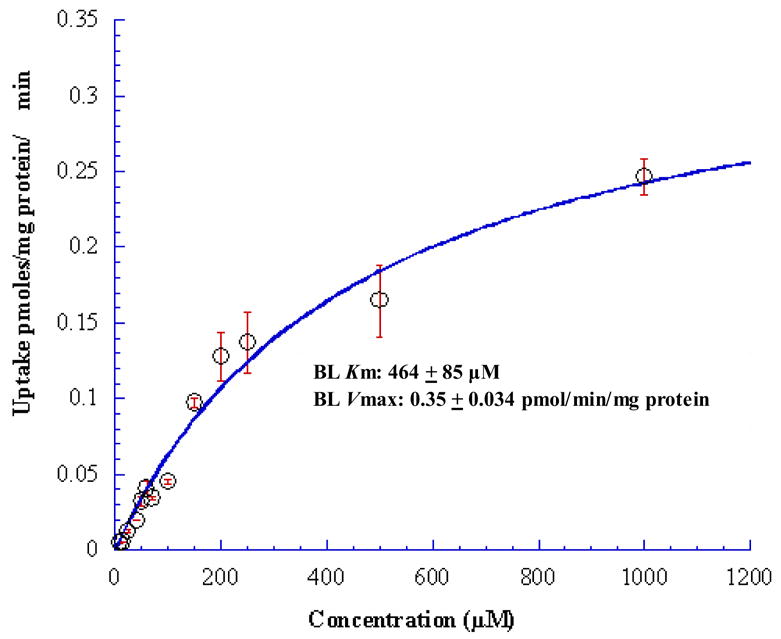
Concentration dependent BL uptake of [3H] Gly-Sar across MDCKII-MDR1 cells. Solid line represents the calculated fit of the data to Michaelis-Menten eq. as described under Materials and Methods. Each data point represents the mean ± S.D. of 4–5 separate uptake determinations
3.3 Time in culture dependent Gly-Sar transport by the AP and BL peptide transporters in MDCKII-MDR1 cells
Figure 4 shows the time in culture dependence of [3H] Gly-Sar transport across the AP and BL peptide transporters in MDCKII-MDR1 cells. There was no statistical difference in the trans-epithelial permeability of [3H] Gly-Sar across both the AP and BL membranes respectively through 4–10 days of culture period. [3H] Gly-Sar transport was inhibited in the presence of unlabeled Gly-Sar in all the studies (data not shown). All subsequent experiments were carried out on the 8th day after seeding to normalize and compare results.
Figure 4.
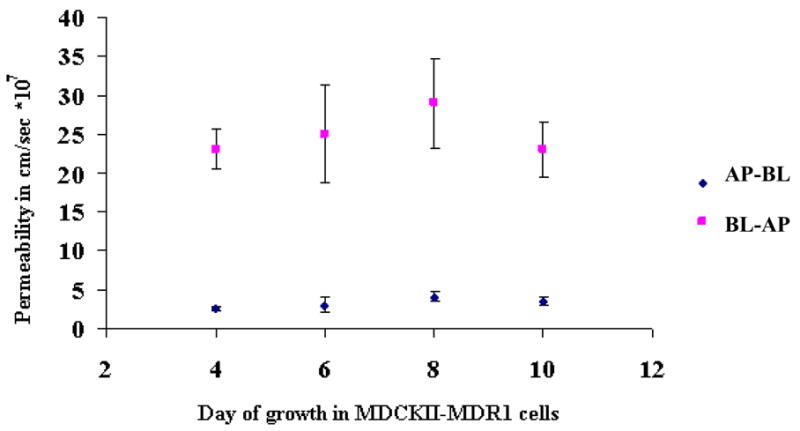
Day dependent AP-BL and BL-AP permeability of [3H] Gly-Sar across MDCKII-MDR1 cells
3.4 Effect of inhibitors on Gly-Sar uptake in MDCKII-MDR1 and MDCKII-WT cells
To determine the substrate specificity, competitive inhibition studies were carried out in the presence of various cephalosporins and ACE-inhibitors. [3H] Gly-Sar uptake by both AP and BL transporters was markedly inhibited by 20 mM of cephalosporins like cefazoline, cephradine, and cefadroxil and ACE-inhibitors like captopril across both the cell lines (Fig. 5).
Figure 5.
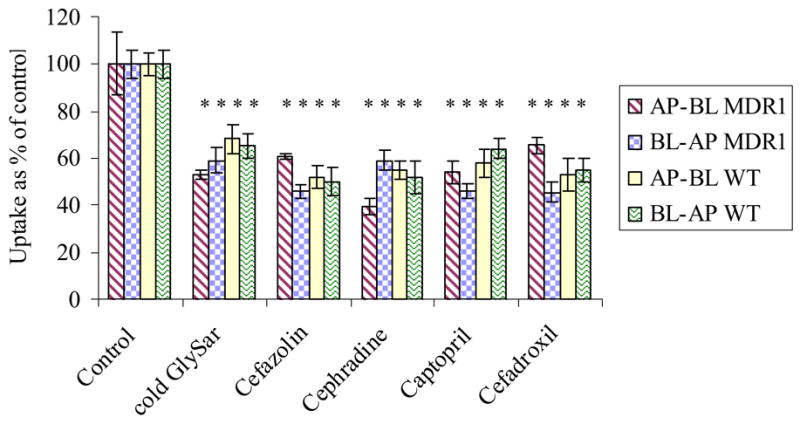
AP and BL uptake of [3H] Gly-Sar in the presence of 20mM inhibitors, cold Gly-Sar, cefazoline, cephradine, cefadroxil, captopril and enalapril across MDCKII-MDR1 and MDCKII-WT cells. Each data point represents the mean ± S.D. of 3–4 separate uptake determinations. Asterisk (*) represents significant difference from control (p <0.05).
3.5 Energy dependence of [3H] Gly-Sar uptake in MDCKII-MDR1 cells
Ouabain, an Na+/K+-ATPase inhibitor, was added at a concentration of 1 mM to delineate energy requirement for [3H] Gly-Sar uptake. Ouabain did not significantly inhibit the uptake of [3H] Gly-Sar from both AP and BL directions indicating that the peptide transport of [3H] Gly-Sar in MDCKII-MDR1 cells is not ATP-dependent (Fig. 6).
Figure 6.
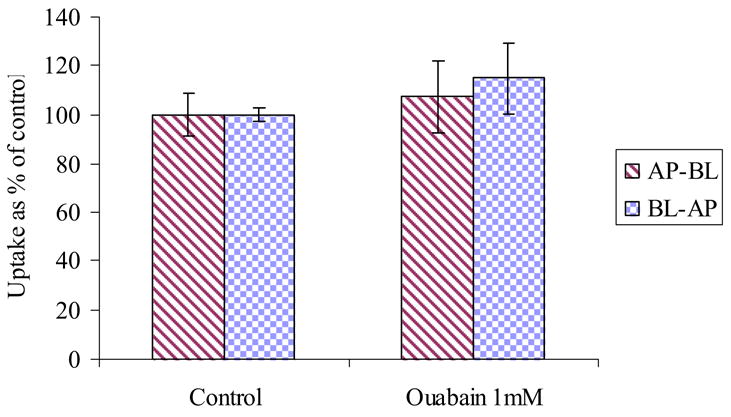
AP and BL uptake of [3H] Gly-Sar in the presence and absence of Ouabain (1mM) across MDCKII-MDR1 cells
3.6 pH dependent transcellular transport of [3H] Gly-Sar in MDCKII-MDR1 cells relative to MDCKII-WT cells
Permeability of [3H] Gly-Sar across both MDCKII-MDR1 and MDCKII-WT cell lines was compared at all the pHs (Table 1). At all pHs examined, [3H] Gly-Sar transport was inhibited in the presence of unlabeled Gly-Sar in both the cell lines (data not shown). BL-AP peptide mediated transport of [3H] Gly-Sar was maximal at pH 4.0 whereas AP-BL peptide mediated transport was maximal at pH 7.4. Also, BL-AP transport of [3H] Gly-Sar was higher than the AP-BL transport of [3H] Gly-Sar at all pHs observed. There is no statistical difference in the BL-AP permeability values of [3H] Gly-Sar in both the cell lines at all observed pHs. However, the AP-BL permeability values of [3H] Gly-Sar in the MDCKII-WT cell line are greater than the AP-BL permeability values of [3H] Gly-Sar in the MDCKII-MDR1 cell line at all pHs.
Table 1.
pH dependent AP-BL and BL-AP transport of [3H] Gly-Sar across MDCKII-MDR1 and MDCKII-WT cells
| MDCKII-MDR1 | MDCKII-MDR1 | MDCKII-WT | MDCKII-WT | |
|---|---|---|---|---|
| AP-BL Permeability of [3H] Gly-Sar *107 cm/sec | BL-AP Permeability of [3H] Gly-Sar *107 cm/sec | AP-BL Permeability of [3H] Gly-Sar *107 cm/sec | BL-AP Permeability of [3H] Gly-Sar *107 cm/sec | |
| pH 4.0 | 3.40 ± 0.15 | 33.2 ± 0.68 | 10.04 ± 1.2 | 28.40 ± 3.0 |
| pH 5.0 | 5.62 ± 0.12 | 17.2 ± 1.86 | 19.76 ± 2.0 | 15.18 ± 1.16 |
| pH 6.0 | 7.42 ± 0.10 | 14.0 ± 0.76 | 20.54 ± 1.25 | 12.31 ± 1.19 |
| pH 7.4 | 16.5 ± 0.23 | 23.7 ± 0.71 | 27.19 ± 2.2 | 23.59 ± 1.45 |
3.7 Transcellular transport of [3H] Gly-Sar in MDCKII-MDR1 cells as compared to Caco-2 cells
Figure 7 represents the comparative permeabilities of [3H] Gly-Sar by AP and BL peptide transporters in Caco-2 at pH 6 and MDCKII-MDR1 cells at all pHs. AP-BL permeability of [3H] Gly-Sar across Caco-2 cells was significantly higher than its AP-BL permeability across MDCKII-MDR1 cells at all pHs. However, BL-AP permeability value of [3H] Gly-Sar across MDCKII-MDR1 at pH 4 (33.2 ± 0.68 * 10−7) was comparable to the AP-BL permeability value of [3H] Gly-Sar across Caco-2 cells at pH 6 (30.82 ± 0.52 * 10−7).
Figure 7.
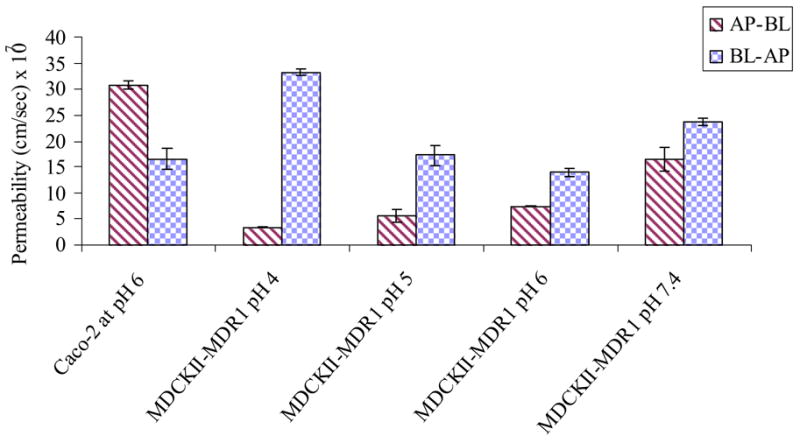
Comparing permeability of [3H] Gly-Sar across Caco-2 at pH 6 and MDCKII-MDR1 cells at all pH 4, 5, 6 and 7.4
4. DISCUSSION
MDCKII-MDR1 cell line was generated by transfecting the human MDR1 gene into MDCK cells. This cell line has been used extensively to study P-gp mediated efflux of various drugs as mentioned earlier. Recently in our laboratory we employed this cell line for studying peptide transporter mediated translocation of several modified compounds which were originally P-gp substrates. These modified compounds were peptide-prodrugs of therapeutic agents which bypassed P-gp mediated efflux and therefore were absorbed intracellularly. A primary objective of this project was to be able to employ the MDCKII-MDR1 cell line in determining simultaneously the affinity of such peptide-prodrugs for both the P-gp and peptide transporters.
Uptake studies in both the AP and BL directions showed a concentration dependent increase in cellular accumulation of [3H] Gly-Sar in MDCKII-MDR1 cells which was saturated at higher concentrations. Apparent Km values for the AP and BL transporters were found to be 457 ± 37 μM and 464 ± 85 μM respectively. Though no statistical differences were observed in the Km values for the AP and BL peptide transporters in MDCKII-MDR1 cell line, the corresponding Vmax values, 0.035 ± 0.001 and 0.35 ± 0.034 pmol/mg protein/min respectively, statistically differed. Thus the capacity of the BL peptide transporter is about 10 times greater than the AP peptide transporter in the MDCKII-MDR1 cell line. The Km and Vmax values for AP and BL peptide transporters for MDCKII-MDR1 cells were significantly different from MDCKII-WT cells indicating that there is a difference in the capacity and the rate of translocation amongst the peptide transporters in the parent and transfected cell line. AP peptide transporters in MDCKII-WT can transport Gly-Sar at a greater rate compared to that in MDCKII-MDR1 cells. However, in MDCKII-MDR1 cells, BL peptide transporters can transport Gly-Sar at a greater rate than AP peptide transporters.
Since Caco-2 cells require 21–28 days of growth in culture for maximal expression of transporters and receptors, we were interested in learning whether there is any functional change in the peptide transporter activity in MDCKII-MDR1 cell line on different days of culture. Transport activities of the AP and BL peptide transporters at pH 7.4 were not growth dependent within 4 to 10 days of culture period. However throughout the culture period, BL-AP transport of [3H] Gly-Sar across MDCKII-MDR1 cell line was constitutively greater than the AP-BL transport. In the MDCKII-WT cells, AP peptide transporter activity was not dependent on culture duration and the BL peptide transporter activity was highest on the 4th and 6th day of culture growth (Terada et al., 2000). But in MDCKII-MDR1 cell line, the AP and BL peptide transporter activity is independent of culture duration through 4–10 days. Thus, there is significant difference between MDCKII-MDR1 and MDCKII-WT cell lines with respect to culture duration based functional peptide transporter activity.
Also, the uptake of [3H] Gly-Sar was inhibited by cephalosporin antibiotics and ACE-inhibitors in both AP and BL directions. Percent inhibition observed for these substrates compared to the control ranged from 40–60% for both the AP and BL peptide transporters. This substrate specificity was found to be similar to that of the MDCKII-WT cells. Uptake studies were also carried out in the presence of Ouabain, a Na+/K+-ATPase inhibitor. No significant inhibition was observed in the uptake of [3H] Gly-Sar in both AP and BL directions. Thus peptide transporter mediated uptake of [3H] Gly-Sar in MDCKII-MDR1 cells does not appear to be energy dependent.
Both AP-BL and BL-AP transport of [3H] Gly-Sar were found to be pH dependent in the MDCKII-MDR1 cell line. BL-AP transport of [3H] Gly-Sar was maximal at pH 4 as compared to pH 5, 6 and 7.4 whereas AP-BL transport was highest at pH 7.4. BL-AP transport of [3H] Gly-Sar across MDCKII-MDR1 cell line was significantly higher than the AP-BL transport at all observed pHs.
Transcellular permeability of [3H] Gly-Sar across MDCKII-MDR1 cells was also compared with MDCKII-WT cells. AP-BL permeability of [3H] Gly-Sar at all pHs observed in MDCKII-WT was significantly greater than that of MDCKII-MDR1 cells. In MDCKII-WT cells, AP peptide transporters relatively transport more peptides than the BL peptide transporters. This pattern has been shifted in MDCKII-MDR1 cells where BL peptide transporters are more efficient in transporting peptides than the AP peptide transporters. We hypothesize that this change in properties of AP peptide transporters in the MDCKII-MDR1 cell line could be due to transfection of the MDCKII-WT cell line with the mdr1 gene. Transfection may have an effect on the expression/functional activity of endogenous transporters like peptide transporters in cells. Further studies including studying functional activity and expression of such endogenous transporters are required to substantiate our assumption.
Hence results presented in this report suggest that although peptide transporters in MDCKII-MDR1 cell line have altered Km and Vmax values as compared to the MDCKII-WT cell line, it still has active peptide transporters on both the apical and the basolateral side, which could be utilized for testing the affinity of substrates for peptide transporters as a rapid screening tool. It can serve as a model for testing peptide mediated drug influx along with its well-proven utility for screening for P-gp mediated drug efflux.
Acknowledgments
This work was supported by National Institute of Health Grants R01 EY 09171-12 and R01 EY 10659-10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Endicott JA, Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;1989;58:137–71. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Guo A, Marinaro W, Hu P, Sinko PJ. Delineating the contribution of secretory transporters in the efflux of etoposide using Madin-Darby canine kidney (MDCK) cells overexpressing P-glycoprotein (Pgp), multidrug resistance-associated protein (MRP1), and canalicular multispecific organic anion transporter (cMOAT) Drug Metab Dispos. 2002;30:457–463. doi: 10.1124/dmd.30.4.457. [DOI] [PubMed] [Google Scholar]
- Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin-Darby canine kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- Ito K, Kusuhara H, Sugiyama Y. Effects of intestinal CYP3A4 and P-glycoprotein on oral drug absorption--theoretical approach. Pharm Res. 1999;16:225–231. doi: 10.1023/a:1018872207437. [DOI] [PubMed] [Google Scholar]
- Jain R, Majumdar S, Nashed Y, Pal D, Mitra AK. Circumventing P-glycoprotein-mediated cellular efflux of quinidine by prodrug derivatization. Mol Pharm. 2004;1:290–299. doi: 10.1021/mp049952s. [DOI] [PubMed] [Google Scholar]
- Jain R, Agarwal S, Majumdar S, Zhu X, Pal D, Mitra AK. Evasion of P-gp mediated cellular efflux and permeability enhancement of HIV-protease inhibitor saquinavir by prodrug modification. Int J Pharm. 2005;303:8–19. doi: 10.1016/j.ijpharm.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Keogh JP, Kunta JR. Development, validation and utility of an in vitro technique for assessment of potential clinical drug-drug interactions involving P-glycoprotein. Eur J Pharm Sci. 2006;27:543–554. doi: 10.1016/j.ejps.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Lown KS, Mayo RR, Leichtman AB, Hsiao HL, Turgeon DK, Schmiedlin-Ren P, Brown MB, Guo W, Rossi SJ, Benet LZ, Watkins PB. Role of intestinal P-glycoprotein (mdr1) in interpatient variation in the oral bioavailability of cyclosporine. Clin Pharmacol Ther. 1997;62:248–260. doi: 10.1016/S0009-9236(97)90027-8. [DOI] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug-herbal interactions. Life Sci. 2006 2006 Mar 27;78(18):2131–45. doi: 10.1016/j.lfs.2005.12.010. Epub 2006 Jan 25. [DOI] [PubMed] [Google Scholar]
- Putnam WS, Ramanathan S, Pan L, Takahashi LH, Benet LZ. Functional characterization of monocarboxylic acid, large neutral amino acid, bile acid and peptide transporters, and P-glycoprotein in MDCK and Caco-2 cells. J Pharm Sci. 2002;91:2622–2635. doi: 10.1002/jps.10264. [DOI] [PubMed] [Google Scholar]
- Rice A, Michaelis ML, Georg G, Liu Y, Turunen B, Audus KL. Overcoming the blood-brain barrier to taxane delivery for neurodegenerative diseases and brain tumors. J Mol Neurosci. 2003;20:339–343. doi: 10.1385/JMN:20:3:339. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Proteau R, Mata JE, Miranda CL, Fan Y, Brown JJ, Buhler DR. Plant polyphenols and multidrug resistance: Effects of dietary flavonoids on drug transporters in Caco-2 and MDCKII-MDR1 cell transport models. Xenobiotica. 2006;36:41–58. doi: 10.1080/00498250500433545. [DOI] [PubMed] [Google Scholar]
- Rouquayrol M, Gaucher B, Roche D, Greiner J, Vierling P. Transepithelial transport of prodrugs of the HIV protease inhibitors saquinavir, indinavir, and nelfinavir across Caco-2 cell monolayers. Pharm Res. 2002;19:1704–1712. doi: 10.1023/a:1020913631309. [DOI] [PubMed] [Google Scholar]
- Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol. 2000;57:679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm Res. 2002;19:765–772. doi: 10.1023/a:1016140429238. [DOI] [PubMed] [Google Scholar]
- Terada T, Sawada K, Ito T, Saito H, Hashimoto Y, Inui K. Functional expression of novel peptide transporter in renal BL membranes. Am J Physiol Renal Physiol. 2000;279:F851–857. doi: 10.1152/ajprenal.2000.279.5.F851. [DOI] [PubMed] [Google Scholar]
- Varma MV, Ashokraj Y, Dey CS, Panchagnula R. P-glycoprotein inhibitors and their screening: a perspective from bioavailability enhancement. Pharmacol Res. 2003;48:347–359. doi: 10.1016/s1043-6618(03)00158-0. [DOI] [PubMed] [Google Scholar]
- Watkins PB. The barrier function of CYP3A4 and P-glycoprotein in the small bowel. Adv Drug Deliv Rev. 1997;27:161–170. doi: 10.1016/s0169-409x(97)00041-0. [DOI] [PubMed] [Google Scholar]
- Williams GC, Knipp GT, Sinko PJ. The effect of cell culture conditions on saquinavir transport through, and interactions with, MDCKII cells overexpressing hMDR1. J Pharm Sci. 2003;92:1957–1967. doi: 10.1002/jps.10458. [DOI] [PubMed] [Google Scholar]


