Abstract
The purpose of this study was to examine the effects of smokeless tobacco (ST) brand switching on biomarkers of ST exposure and on ST use. Subjects seeking treatment to reduce their use were randomized to ST brand switching with controlled ST topography, brand switching with ad libitum ST use, or a waitlist control with subsequent randomization to one of these two conditions. The waitlist control group was included to assess whether changes were a consequence of time effect. During the intervention, Copenhagen or Kodiak ST users were asked to switch to products that were sequentially lower in nicotine content: Skoal Long Cut Original or Wintergreen for 4 weeks and then Skoal Bandits for the subsequent 4 weeks. Measures were obtained during the course of treatment and at 12-week follow-up. Significant reductions in total urinary cotinine and 4-(methylnitrosamino)-l-(3 pyridyl) l-butanol (NNAL) plus its glucuronides (total NNAL) were observed with no significant differences between the controlled topography and ad libitum conditions. Significant reductions were also observed in the amount and duration of dips with a significant intervention effect for durational measures. At 12 weeks, the 7-day biochemically-verified tobacco abstinent rate was 26% in the ad libitum group. ST brand switching may be a feasible alternative intervention for ST users interested in quitting but unwilling to stop ST use completely.
Keywords: Smokeless tobacco, brand switching, toxicant exposure, tobacco use cessation
1. Introduction
Reduction in exposure to tobacco-associated toxicants as a means to reduce morbidity and mortality may be an alternative to cessation among tobacco users unable or unwilling to quit (Stratton et al., 2001). Furthermore, a systematic reduction in nicotine levels of the tobacco product may facilitate tobacco abstinence. One method to reduce nicotine intake and toxicant exposure is switching to tobacco brands that contain lower concentrations of these constituents. This approach allows for gradual nicotine weaning while maintaining the sensory and habit aspects of tobacco use. Brand switching, which has been used with cigarette smokers as a method to prepare for cessation or as a method to reduce nicotine and tar levels, has demonstrated varying success (Fiore et al., 2000; Foxx and Brown, 1979; Glasgow et al., 1983; Prue et al., 1981). One problem associated with brand switching among cigarette smokers has been the occurrence of compensatory smoking, that is, smoking more in order to attain the nicotine levels achieved with their usual cigarette brand (National Cancer Institute, 2001; U.S. Department of Health and Human Services, 1996). Evidence exists clearly demonstrating that switching to “ultra-light” and “light” cigarettes leads to compensatory smoking behavior and, consequently, to no reduction in harm (Hecht et al., 2005; National Cancer Institute, 2001). Furthermore, many of these “light” cigarette smokers believed that they were reducing harm which may have undermined cessation attempts (Cohen, 1996; Etter et al., 2003; Kozlowski et al., 1998; Shiffman et al., 2001). While brand switching has been a recommended treatment approach for smokeless tobacco (ST) users (Severson and Hatsukami, 1999), the effects of brand switching in this population of tobacco users has not been systematically explored.
The primary goal of this study was to examine the effects of brand switching on levels of nicotine intake and toxicant exposure among ST users. In addition, we determined the extent to which ST users are able to successfully switch to brands that contained about 50% of the nicotine content of their usual brand followed by brands that contained less than 25% of the nicotine content of their usual brand. Finally, the percentage of ST users who make > 24 hour quit attempt(s) and who become abstinent (7-day point prevalence tobacco abstinence) was assessed. In this study, subjects were randomly assigned to: (1) brand switching and controlled ST topography with instructions not to increase the number of dips per day, amount of dip or duration of dip per day; (2) brand switching with ad libitum ST use; or (3) waitlist control that maintained use of their normal ST brand to determine the stability of our measures over time. The condition controlling ST topography was incorporated in order to determine whether compensation can be minimized. We hypothesized that: a) a significant reduction in nicotine intake would be observed with brand switching compared to usual ST brand use; b) a significant reduction in toxicant exposure would be observed with brand switching compared to use of the usual ST brand; c) the brand switching with controlled ST topography group would have a greater reduction in nicotine and toxicant exposure than the brand switching with ad libitum ST use group; and d) brand-switching would not deter quit attempts.
2. Methods
2.1 Subject recruitment
Potential subjects were recruited from the Minneapolis, MN metropolitan area through newspapers and radio advertisements and screened over the telephone to determine interest and eligibility. Subjects were eligible to participate if they were: a) between 18 and 70 years of age; b) interested in reducing ST use but not quitting (having an established quit date) within the next 90 days; c) used ST daily (≥ 6 dips per day) for the past six months, d) in good physical health (no unstable medical condition or medication use that might affect tobacco use or be affected by tobacco use reduction), and in good mental health (e.g. not currently, within past 6 months, taking psychotropic medications or having a psychiatric diagnosis, including substance abuse, as determined by DSM-IV criteria). The specified rate of tobacco use as an inclusion criterion was based on being able to target heavy ST users so that an actual reduction in toxicant exposure would be observed. This number was also based on prior studies that have observed that the mean number of dips used per day for ST users seeking cessation treatment was 6 to 10 (Hatsukami and Severson, 1999). All subjects were selected based upon their use of Copenhagen or Kodiak Wintergreen so that adequate reduction in the nicotine levels of ST would be achieved. Both of these products have equivalent amounts of nicotine content, pH and percent free nicotine (Djordjevic et al., 1995). Subjects were also excluded if they used alternate tobacco or nicotine products or were pregnant or nursing.
Potential subjects were invited to attend an orientation and screening visit. During this visit, informed consent was obtained and subjects were informed that the study would compare different intervention approaches to examine the effects of switching to ST brands with lower concentrations of nicotine and toxicants. Enrolled subjects completed a ST use questionnaire, medical history form, and baseline measurements which included all outcome measures.
2.2 Experimental procedure
After study enrollment, subjects were required to monitor their ST use on a daily basis for a period of two weeks. Subjects received a handheld computer on which they recorded the number and duration of ST dips. The data collected from this monitoring process helped determine baseline level and topographical features of use (e.g., dips per day, duration of dip). Subjects were then randomly assigned to one of three conditions: 1) brand switching with controlled ST topography; 2) brand switching with ad libitum ST use; or 3) waitlist control.
Subjects assigned to the brand switching conditions were asked to use ST brands that reduced free nicotine concentrations by about 50% during the first four weeks (Skoal Long Cut Straight or Wintergreen) and greater than 75% in the second four weeks (Skoal Bandits) compared to the ST brand typically used. Skoal Long Cut Straight and Wintergreen are loose leaf tobacco products with free nicotine content ranging from 2.4 to 3.7 and 2.0 to 4.1 mg/g, respectively (Centers for Disease Control and Prevention, 1999; Henningfield et al., 1995; Richter and Spierto, 2003). For Skoal Long Cut Straight, the total tobacco-specific nitrosamine level is 9.2 μg/g product wet weight and the tobacco-specific carcinogen 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone (NNK) level is 0.47 μg/g; no values are available for Skoal Long Cut Wintergreen (Stepanov et al., 2006). Skoal Bandits are dispensed in packets that contain 0.5 g of ST. The free nicotine content for this product ranges from 0.02 to 0.97 mg/g for original, wintergreen and mint (Centers for Disease Control and Prevention, 1999; Djordjevic et al., 1995; Henningfield et al., 1995; Richter and Spierto, 2003). The total tobacco-specific nitrosamine level is 1.3 μg/g product wet weight and the NNK level is 0.2 μg/g (Stepanov et al., 2006). These values are compared to free nicotine content of 3.1 to 9.0 mg/g for Copenhagen snuff and 5.8 to 6.1 for Kodiak Wintergreen (Centers for Disease Control and Prevention, 1999; Djordjevic et al., 1995; Henningfield et al., 1995; Richter and Spierto, 2003). The respective total tobacco-specific nitrosamines values are 4.8 μg/g and 4.5 μg/g and NNK values are 0.8 μg/g and 0.4 μg/g (Stepanov et al., 2006).
Subjects assigned to the controlled topography group were informed that they were to use no more than the mean number of dips and tins used during the baseline session and no longer than the mean duration of use. Data collected on ST topography during the 2-week baseline period was used to determine these parameters. Subjects in this condition were cued for the duration of each dip by using the computerized device programmed to indicate how long to keep the dip in their mouths. This device recorded the number of dips and the time and duration of each dip used during the course of the day. However, because of technological difficulties associated with this device (e.g., short battery life, missing data), subjects were also asked to record the frequency and duration of each dip on a tobacco use diary card while adhering to the required tobacco use parameters. In the brand switching with ad libitum ST use condition, subjects were allowed to use the different brands of ST on an ad libitum, non-controlled basis. They also were required to use the computer device and to self-report ST use on the tobacco use diary cards. In both conditions, subjects were provided the tin(s) of ST. All used and unused tins were returned at each clinic visit. These tins were weighed prior to dispensing and when they were returned to the clinic. For the Skoal Bandits, the number of pouches was counted. Although subjects agreed not to use products other than those assigned to them for the first 12 weeks of the study, subjects were asked to record any dips that were taken of unassigned brands or any other nicotine or tobacco product on the tobacco use diary card.
Subjects attended clinic visits weekly for a period of 8 weeks during which time outcome measures were obtained and behavioral counseling was provided. These individual sessions lasted no more than 10 minutes. During these sessions, a specific format was followed including: a) asking about tobacco use status; b) discussing motivations for nicotine reduction; c) discussing any problems encountered; d) problem solving difficult situations or issues of compliance; and e) providing support.
At the end of the 8-week treatment period, subjects were asked if they would like to quit. If so, they set a quit date and follow-up calls were conducted one week after their quit date. ST users who wanted to quit prior to the end of treatment received encouragement to make a quit attempt. Counseling was provided and involved: a) discussing reasons for wanting to quit and potential obstacles; b) discussing the content of the treatment manual, Tough Enough to Quit Snuff, which included information on preparation for the quit day, identification of high risk situations, and strategies to deal with these situations; and c) identifying sources of support. Participants who did not want to quit were encouraged to maintain reduction or reduce even further. For both quitters and reducers, subjects were provided the opportunity to receive Skoal Bandits for another 4 weeks.
A follow-up session occurred at 12 weeks after the initiation of the study. Every attempt was made to follow all subjects even if they were unable to adhere to brand switching. Subjects were required to monitor the amount of ST and other tobacco use for the one-week prior to the follow-up clinic visit using daily diary cards, which were dispensed at the 8-week visit. Other outcome measures were also assessed at this time.
The third condition was a waitlist control group who was informed to continue using their usual brand and amount for 10 weeks. They only attended sessions that involved collection of biomarkers (Baseline, Weeks 4 and 8) and were paid for their attendance. After completing this phase of the study, they were then randomized to one of the brand switching conditions.
2.3 Measures
Subjects were assessed for the following outcome measures during each clinic visit: tobacco use status (which included the assessment of the use of other tobacco and medicinal nicotine products), craving and withdrawal symptoms as measured by the Minnesota Nicotine Withdrawal Scale (Hughes and Hatsukami, 1998; Hughes and Hatsukami, 1986), vital signs (heart rate and blood pressure), weight, and expired carbon monoxide using a Bedfont Micro Smokerlyzer® (manufactured in UK) measurement device. Other measures such as the Contemplation Ladder (assesses interest in quitting using an image of a 11 rung ladder with rungs labeled 0=not thought of quitting, 5=thinking I should quit, but not ready, to 10= taking action to quit; Biener and Abrams, 1991), and Perceived Health Risk (10 items listing medical symptoms and disease, rated on a 0-10 scale, with higher scores reflecting greater health risk) were measured at the screening visit, once during baseline prior to the reduction phase and at 4, 8 and 12 weeks. The extent to which the participants swallowed the tobacco juice (never, sometimes or always) was assessed at baseline, 4 and 8 weeks and their satisfaction with the product (dissatisfied, neutral or satisfied) at 4 and 8 weeks.
Urine samples were collected twice during baseline use and at the end of 4 and 8 weeks to assess for cotinine and 4-(methylnitrosamino)-l-(3 pyridyl) l-butanol (NNAL) plus its glucuronides (total NNAL). NNAL and its glucuronides are metabolites of the tobacco-specific carcinogen NNK. Urinary total cotinine concentrations were determined by gas chromatography/mass spectrometry (GC/MS) as previously described (Hecht et al., 1999). Analysis for total NNAL was conducted as previously described (Carmella et al., 2003).
2.4 Compliance
Compliance with session attendance was maximized by paying subjects $200 at the end of treatment if they attended all sessions. In addition, subjects were paid for the subsequent follow-up clinic visit ($25).
2.5 Statistical analysis
Concentrations of total cotinine and total NNAL were analyzed using repeated measures linear mixed models to investigate whether the reduction strategies have different effects on the extent of tobacco exposure reduction and whether changes were also observed in the waitlist group (Cnaan et al., 1997; Littell et al., 2000; Schluchter, 1988). Time effect was the primary goal for analysis in the waitlist group. For comparison of the two experimental conditions, the analysis modeled the change from baseline in ST exposure at each visit (week 4 and 8) as a function of treatment and the time of visit. The models included both main effects for the week of study or time and treatment condition as well as interactions between the treatment conditions and time of study. The variance-covariance structure was selected as compound symmetry based on the likelihood ratio test and Akaike Information Criterion. The variance components were estimated using the method of restricted maximum likelihood (REML). The denominator degrees of freedom for the tests of the main effects were computed by using a general Satterthwaite approximation. The multiple comparisons of the least squares mean (LS-means) for the main effects were conducted with Tukey adjustment. The F-test was used to determine main effects for time and for treatment conditions, and for treatment by time interactions. Analyses of total cotinine and total NNAL were also extended to 12 weeks.
Similar repeated measures models were used to investigate the effects of the two reduction strategies on each of the other outcome measures over the course of 8 weeks of treatment and extended to 12 weeks. These outcomes measures included quantitative measures of ST use over the course of the 8 weeks and included: dips per day; tins per week; duration per dip; dips per week x duration per dip (total duration). Dips per day and tins per week at 12 weeks were also analyzed. Other measures included heart rate, blood pressure, weight, perceived health risk, motivation to quit, withdrawal symptoms, and extent of swallowing the tobacco juice.
In addition, the two reduction strategies were compared on number of > 24 hour quit attempts, 7-day point prevalence tobacco abstinence, and longest duration of abstinence. The 7-day point prevalence tobacco abstinence rate was biochemically-verified using a urinary cotinine concentration of less than 100 ng/mL (Moyer et al., 2002) Rates of abstinence attempts and past week abstinence were assessed for weeks 1-4, 5-8 and 9-12. The Chi-square statistic was used to test significance of these outcome measures and t-statistic for the duration of abstinence.
3. Results
3.1 Subject characteristics
Of the 226 potential subjects screened over the telephone, 148 were considered eligible to attend the orientation meeting. ST users were considered ineligible due to the following criteria: insufficient use of smokeless tobacco (N=17); not using Kodiak or Copenhagen (N=24); significant or recent health or psychiatric problems (N=5); excessive use of alcohol or other tobacco products (N=11); smoking greater than 10 cigarettes per month (N=8); or multiple combinations of the listed reasons for exclusion (N=13). Of the eligible ST users, 88 attended the orientation meeting and signed informed consent. Sixty-six subjects were randomized to treatment with 35 in the controlled topography group and 31 in the ad libitum group. Of these subjects, 7 who were randomized to the controlled topography group and 6 to the ad libitum group were initially assigned to the waitlist control. Of those subjects randomized to treatment, 11 dropped out of the study, 8 from the controlled topography group (2 during the waitlist period, and 1 each during baseline, and weeks 1, 2, 3, 4, 5) and 3 from the ad libitum group (2 during waitlist and 1 during baseline). The high rate of drop-outs in the controlled topography group may have been a function of the mechanical problems experienced with the computer devices and their labor intensiveness.
The demographics and tobacco use history of the sample randomized to treatment are described in Table 1. No statistically significant differences were observed for any of the variables.
Table 1.
Baseline demographics and tobacco use history in a study of smokeless tobacco brand switching comparing controlled ST topography use and ad libitum use (N = 66)
| Controlled topography (N = 35) | Ad libitum use (N = 31) | ||
|---|---|---|---|
| Variables | Mean (SE) | Mean (SE) | p-value |
| Age (years) | 33.2 (6.98) | 31.8 (5.05) | 0.35 |
| Sex (%male) | 100% | 100% | |
| Duration of use (months) | 70.5 (30.6) | 60.0 (32.4) | 0.18 |
| Modified FTND* | 7.3 (1.64) | 7.5 (1.79) | 0.59 |
| Brand of smokeless (%Kodiak) | 43% | 61% | 0.15 |
| Age of first use (years) | 16.4 (3.1) | 15.8 (4.6) | 0.51 |
| Age of daily use (years) | 18.8 (4.2) | 19.2 (5.4) | 0.74 |
| Tins per week | 3.7 (1.6) | 4.2 (1.9) | 0.31 |
Modified Fagerström Tolerance Questionnaire for smokeless tobacco users (Boyle et al., 1995)
3.2 Waitlist group
To determine stability of measures for exposure biomarkers and amount of ST use, the waitlist control group data was analyzed for these variables over the 8 week period. No significant changes were observed in tins per week (F=0.29, p=0.59), dips per day (F=0.00, p=0.97), total cotinine (F=1.50, p=0.22) and total NNAL concentrations (F=0.55, p=0.46). These results would indicate that any changes observed during the treatment phase would not be a function of time effects.
3.3 Treatment compliance
The percent compliance in use of assigned brands was 98% for Skoal and 98% for Skoal Bandits in the controlled topography group and 100% for Skoal and 99% for Skoal Bandits in the ad libitum group during treatment. Compliance with ST as the only tobacco use was determined by measurement of weekly carbon monoxide levels. The mean carbon monoxide levels for each week ranged from 1.6 to 2.9 parts per million (ppm). Only two subjects exceeded the cutoff of 8 ppm suggesting cigarette smoking. One subject had worked in a machine shop (CO = 11 ppm at week 4) and the other subject denied any inhaled tobacco use (CO = 13 at week 12).
3.4 Biomarkers for ST exposure
With brand switching, significant changes were observed in biomarkers for exposure during the 8 week treatment phase (Figure 1). Significant time effects were observed for mean cotinine concentrations (F= 35.65, p < 0.0001) with greater reductions occurring over the treatment period. Significant differences were observed between baseline (week 0) and week 8 (p < 0.0001) and between week 4 and week 8 (p < 0.0001) but not between baseline and week 4 (p = 0.15). No treatment (p = 0.15) nor treatment by time effects (p = 0.66) were observed. Significant time effects were also observed for mean total NNAL concentrations (F = 16.38, p <0.0001), with reduction in levels over the treatment period. Significant differences were observed between baseline and week 8 (p < 0.0001), week 4 and week 8 (p < 0.0001), but not between baseline and week 4 (p = 0.44). No significant treatment (p = 0.68) and time by treatment effects (p = 0.70) were observed.
Figure 1.
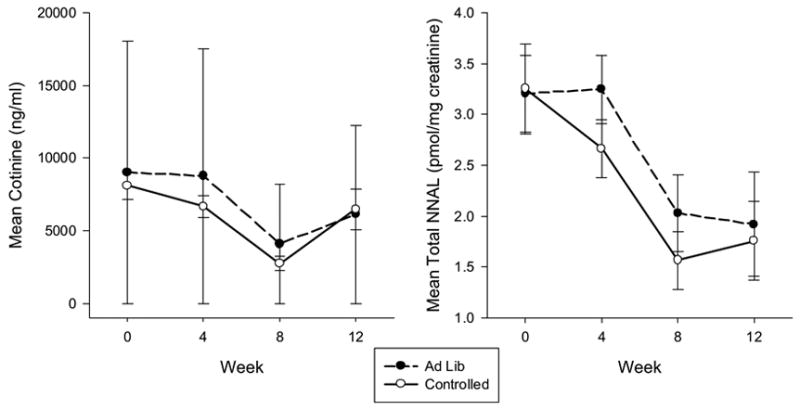
Mean cotinine and total NNAL concentrations over time across experimental conditions during treatment and at the 12 week follow-up.
3.5 Amount of ST use
Along with reductions in ST biomarkers, reductions were observed in the amount of ST use (Figure 2). Significant time effects were observed for self-reported mean dips per day (F = 20.02, p < 0.0001) with greater reductions over time. Significant differences were observed between baseline and week 8 (p <0.0001), week 4 and week 8 (p = 0.001), and baseline and week 4 (p = 0.003). No treatment (p = 0.60) nor treatment by time effects (p = 0.51) were observed for mean dips per day. Significant time effects were observed for mean tins per week (F= 30.14, p < 0.0001). Significant differences were observed between baseline and week 8 (p < 0.0001) and between week 4 and week 8 (p < 0.0001) for mean tins per week, but not between baseline and week 4 (p = 0.23). A near significant treatment effect (F = 3.56, p = 0.061) was observed, with ST users in the ad libitum group using more mean tins per week than the controlled topography group up to week 12, but no significant treatment by time effects were observed for mean tins per week (p = 0.25).
Figure 2.
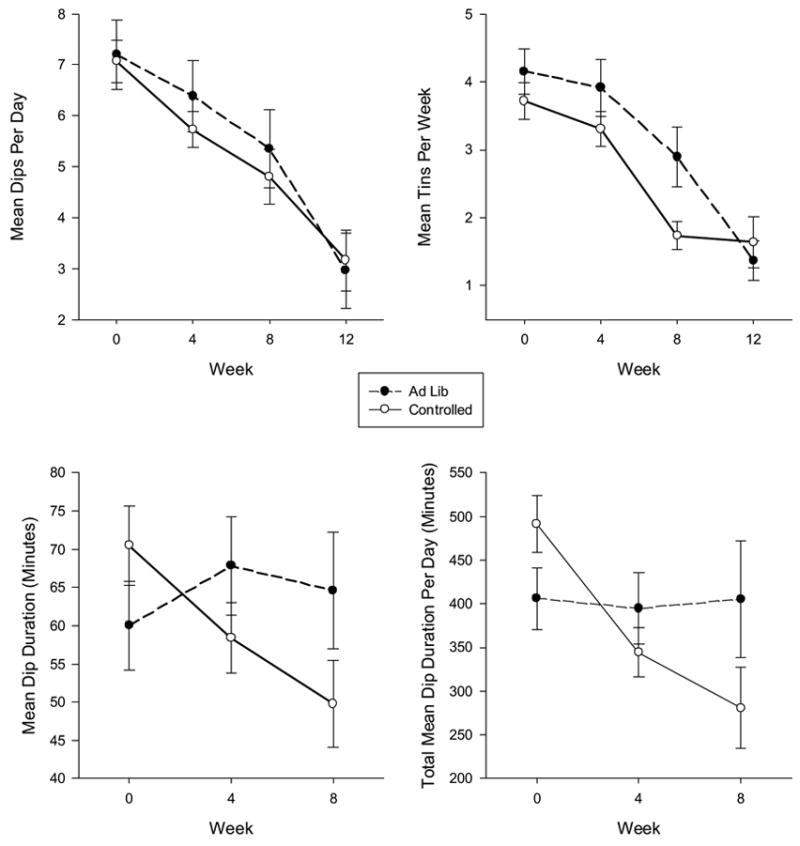
Mean dips per day, tins per week, dip duration, and total mean dip duration per day over time across experimental conditions during treatment and at follow-up for the quantity and frequency measures.
Significant time (F = 4.60, p = 0.012) and significant treatment by time effects (F = 5.95, p = 0.004) but no significant treatment effect (p = 0.79) were observed for mean dip duration. ST users in the controlled topography condition showed a decrease in dip duration with relatively no change in the ad libitum group. Significant time (F= 14.58, p <0.0001) and treatment by time effects (F = 9.09, p < 0.001) were also observed for total mean dip duration. ST users in the controlled topography condition showed a decrease in total mean dip duration with relatively no change in the ad libitum group. No significant treatment effect (p = 0.77) was found.
At the 12 week follow-up, significant reductions continued to be observed compared to baseline for mean cotinine concentrations (t = −2.16, p = 0.035), total NNAL (t = −3.24, p = 0.002), mean tins per week (t = −7.74, p < 0.0001), and mean dips per day (t = −6.40, p < 0.001). Duration of use could not be calculated because of too many missing values. No treatment effects were observed at week 12.
3.6 Correlation between amount of ST use and biomarker measures
A significant correlation was observed between cotinine concentrations and tins per week (r = 0.29, p = 0.039), but when controlling for amount of use (tins per week), no significant brand effects were observed. No significant correlation was observed for tins per week and total NNAL concentrations (r=0.09, p=0.51).
3.7 Reduction in physiological responses (heart rate, blood pressure and weight)
No significant time or treatment effects were observed for heart rate, blood pressure or weight (p > 0.10) through the 12 week follow-up.
3.8 Reduction in subjective responses
Significant time effect (F = 19.37, p <0.0001) was observed on the contemplation ladder. Significant differences were observed between baseline and week 4 (p < 0.0001) and between baseline and week 8 (p < 0.0001) with increasing motivation to quit over time. No significant treatment (p=0.89) and time by treatment interaction was observed (p =0.35). Time effect was significant for perceived health risk (F=81.89, p < 0.0001) with increasing perceived health risk over time. Significant differences were observed from baseline to week 4 (p<0.0001), baseline to week 8 (p<0.0001) and between weeks 4 and 8 (p = 0.005).
No time effects, treatment effect and time by treatment effect were significant for withdrawal symptoms (ps > 0.05). Only time effect was observed for craving (F=4.38, p = 0.015) with craving reducing over time. Significant differences were observed from baseline to week 8 (p=0.004). No significant difference were observed from baseline to week 4 (p=0.21) and between weeks 4 and 8 (p =0.09).
At the 12 week follow-up, a significantly higher perceived health risk continued to be observed compared to baseline (t = 3.48, p = 0.001), but no significant differences were observed for motivation to quit (t = 0.95, p = 0.35), craving (t = −1.95, p = 0.06), and withdrawal symptoms (t = 0.68, p= 0.50).
No significant changes in amount of tobacco juice swallowed were observed when switched from own brand and Skoal Long Cut (p =.80), from own brand to Skoal Bandits (p = 0.55) or from Skoal Long Cut to Skoal Bandits (p = 0.74). With drop-outs considered as not satisfied with the products, the percent who indicated that they were dissatisfied with the Skoal Long Cut was 32% and with Skoal Bandits was 44%.
3.9 Quit attempts and duration of abstinence
Significant time effects were observed for the percentage of subjects reporting 24 hour quit attempts ( , p = 0.007) with increasing number of attempts over time. Significant differences were observed between week 4 and week 8 (p = 0.038) and between week 4 and week 12 (p = 0.006), but not between week 8 and week 12 (p = 0.55). No significant treatment (p = 0.19) or treatment by time effects (p = 0.51) were observed for the percentage of subjects reporting 24 hour quit attempts.
Significant time effects were observed for 7-day point prevalence abstinence ( , p = 0.001). Significant differences were observed between week 4 and week 12 (p < 0.001) and between week 8 and week 12 (p = 0.026), but not between week 4 and week 8 (p = 0.09). No significant treatment (p = 1.00) or treatment by time effects (p = 0.40) were observed for the 7-day point prevalence tobacco abstinence rate (Table 2).
Table 2.
Quit attempts and biochemically-verified abstinence in a study of smokeless tobacco brand switching comparing controlled ST topography use and ad libitum use (N = 66)
| Outcome | Week 1–4 | Week 5–8 | Week 9–12 | |||
|---|---|---|---|---|---|---|
| Controlled (%) | Ad libitum (%) | Controlled (%) | Ad libitum (%) | Controlled (%) | Ad libitum (%) | |
| Quit attempt >=24 hours | 2.8 | 9.7 | 25.7 | 25.8 | 29.0 | 48.3 |
| Quit attempt >=7 days | 0.0 | 0.0 | 0.0 | 6.5 | 17.1 | 25.8 |
No significant differences were observed in longest duration of abstinence between the ad libitum and controlled topography group (12.36 days vs 12.38 days, t =-0.01, p = 0.99).
3.10 Use of products during follow-up
Of the subjects within the ad libitum condition who continued to use products at the 12-week follow-up and did not drop out, 15 reported use of Skoal Bandits solely, 2 used Skoal Bandits primarily, and 3 used other products primarily or solely. Within the controlled use condition, 7 reported use of Skoal Bandits solely, 4 used Skoal Bandits primarily, and 7 used other products primarily or solely.
4. Discussion
We observed that ST brand switching leads to a significant reduction in total mean cotinine and NNAL concentrations among ST users interested in reducing ST use. Both the controlled topography and ad libitum groups demonstrated a significant reduction in amount of tobacco use as measured by dips per day and tins per week. At week 12, the biochemically-verified 7-day point-prevalent tobacco abstinent rate was 26% in the ad libitum group.
One of the major strengths of our study relates to the use of a waitlist control group. The waitlist control allowed for the assessment of a potential time effect on the observed changes in the controlled topography and ad libitum groups. Controlling for a time effect is important when conducting ST research since significant variability in bioavailable nicotine exists between cans of the same ST brand due to differences in chemical composition between batches and conditions of storage (Djordjevic et al., 1995). Furthermore, the concentrations of toxicants in ST manufactured in the United States increases significantly over time when stored (Brunnemann et al., 2001; Foulds et al., 2003). We observed no time effect in the waitlist group, providing validity to our observation of a reduction in total mean cotinine and NNAL concentrations with brand switching.
We observed a significant relationship between amounts of ST use with cotinine concentrations, suggesting that the observed reduction in total cotinine concentrations may be related to both a reduction in amounts of ST use and the switching to brands with lower nicotine levels. However, we did not observe a relationship between amounts of ST use and total NNAL concentrations, suggesting that reductions in total NNAL are related to a reduction of NNK in the ST brands. Based upon prior studies, the NNK levels in Skoal Bandits (1.3 μg/g) is significantly lower than the most popular ST brands such as Copenhagen (4.8 μg/g) (Stepanov et al., 2006), although these level can vary depending on time and storage conditions as well as by batch. The levels of total NNAL reduced by brand switching in the current study was from a mean of 3.2 pmol/mg creatinine to 1.8 pmol/mg creatinine, or over a 50% reduction. This level of reduction is comparable to reductions observed when ST users are switched from Copenhagen and Skoal to a Swedish snus product (Hatsukami et al., 2004). Swedish studies suggest that the head and neck cancer risk with the use of snus is not significantly elevated compared to non ST users (Lewin et al., 1998; Schildt et al., 1998). However, the manufacturing process of ST products in the U.S. is fundamentally different from that of snus in Sweden (Foulds et al., 2003), and further investigation is required to determine if a reduction in NNK exposure with U.S. ST brand switching results in a cancer risk reduction. Available evidence suggests that snus use may be associated with an increased risk for cardiovascular events (Foulds et al., 2003) and pancreatic cancer (Boffetta et al., 2005); therefore, ST products with lower concentrations of toxicants, such as snus, should not be considered safe.
Our findings suggest that ST brand switching may not be comparable to brand switching for cigarette smoking. The key difference likely resides in the nicotine delivery methods of the two tobacco products and the ability to compensate for changes in nicotine yield. While virtually any brand of cigarette can provide the user with the desired dosage due to changes in smoking patterns, the dose obtained from ST is determined by the product itself (Henningfield et al., 1995). Cigarette smokers who are switched to cigarettes with lower nicotine yields by Federal Trade Commission (FTC) criteria are known to compensate by changing their smoking topography (i.e., increases in puffing intensity and volume) or blocking ventilated filters (Scherer, 1999) resulting in little change in salivary cotinine concentrations (Hammond et al., 2005). Furthermore, cigarette brand switching has not been observed to be a significant predictor for smoking abstinence (Haddock et al., 1999) and is not a recommended approach for smoking cessation (Fiore et al., 2000). In the present study, we observed an overall decrease in the number of dips and the tins consumed, not a compensatory increase, in the ad libitum group with ST brand switching. In a post-hoc analysis of week by week data, no significant increase in dips per day or tins per week was seen even during the one week following the switch in brands. Furthermore, the findings suggest that controlling topography, while reducing duration of ST use and lower amounts of tins per week, has no added effect for reducing total cotinine or NNAL concentrations compared to the ad libitum group. Given that controlling topography is labor-intensive and resulted in a high attrition rate, particularly when using computerized devices, instructing ST users about the potential of compensation may be sufficient when recommending this approach clinically.
The abstinence rates observed in the current study are comparable to those observed in previous ST cessation studies among patients receiving behavioral support alone (Dale et al., 2002). We observed that at week 12, up to 26% of subjects in this group were biochemically-verified point prevalent abstinent. And while the ST users in this study were interested in reducing ST use but had not selected a quit date, our findings support the clinical recommendation of this strategy for ST users interested in quitting tobacco but not stopping tobacco completely (Severson and Hatsukami, 1999), .
Several limitations to this study exist. First, our study included a short duration of ST brand use. The duration of time that ST users will comply or sustain use of ST brands with lower nicotine concentrations is unknown. Second, this study had a small sample size and results may not be generalizable to a larger population of ST users. Finally, this study involved the use of a particular brand of ST during brand switching. In a more naturalistic setting, significantly reduced toxicant exposure may not be observed if ST users concomitantly use their own brand with brands containing lower toxicant levels.
In summary, ST brand switching reduces total cotinine and NNAL concentrations among ST users interested in reducing use. The unique properties of ST and the abstinence rates observed in our study support the clinical recommendation for brand switching as a treatment approach for ST users. Future investigations into the optimal brand switching regimen are warranted.
Acknowledgments
This study was supported by R01 DA14404 and P50 DA013333
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Biener L, Abrams D. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Aagnes B, Weiderpass E, Andersen A. Smokeless tobacco use and risk of cancer of the pancreas and other organs. Int J Cancer. 2005;114:992–995. doi: 10.1002/ijc.20811. [DOI] [PubMed] [Google Scholar]
- Boyle RG, Jensen J, Hatsukami DK, Severson H. Measuring dependence in smokeless tobacco users. Addict Behav. 1995;20:443–450. doi: 10.1016/0306-4603(95)00013-3. [DOI] [PubMed] [Google Scholar]
- Brunnemann KD, Qi J, Hoffman D. Aging of oral moist snuff and the yields of tobacco specific n-nitrosamines (TSNA) Boston, MA: 2001. [Google Scholar]
- Carmella SG, Han S, Fristad A, Yang Y, Hecht S. Analysis of total 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human urine. Cancer Epidemiol Biomarkers Prev. 2003;12:1257–1261. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Determination of nicotine, pH, and moisture content of six U.S. commercial moist snuff products - Florida, January-February, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:398–401. [PubMed] [Google Scholar]
- Cnaan A, Laird N, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Cohen J. Smokers' knowledge and understanding of advertised tar numbers: health policy implications. Am J Public Health. 1996;86:18–24. doi: 10.2105/ajph.86.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale LC, Ebbert JO, Schroeder DR, Croghan IT, Rasmussen DF, Trautman JA, Cox LS, Hurt RD. Bupropion for the treatment of nicotine dependence in spit tobacco users: a pilot study. Nicotine Tob Res. 2002;4:267–274. doi: 10.1080/14622200210153821. [DOI] [PubMed] [Google Scholar]
- Djordjevic M, Hoffman D, Glynn T, Connolly G. US commercial brands of moist snuff, 1994. I. Assessment of nicotine, moisture, and pH. Tob Control. 1995;4:62–66. [Google Scholar]
- Etter JF, Kozlowski LT, Perneger TV. What smokers believe about light and ultralight cigarettes. Prev Med. 2003;36:92–98. doi: 10.1006/pmed.2002.1129. [DOI] [PubMed] [Google Scholar]
- Fiore M, Bailey W, Cohen S, Dorfman S, Goldstein M, Gritz E, Heyman R, Jaen C, Kottke T, Lando H, Mecklenburg R, Mullen P, Nett L, Robinson L, Stitzer M, Tommasello A, Villejo L, Wewers M. Clinical practice guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2000. Treating tobacco use and dependence. [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerstrom K. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tob Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxx RM, Brown RA. Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. J Appl Behav Anal. 1979;12:111–125. doi: 10.1901/jaba.1979.12-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Klesges RC, Vasey MW. Controlled smoking for chronic smokers: an extension and replication. Addict Behav. 1983;8:143–150. doi: 10.1016/0306-4603(83)90008-4. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Talcott GW, Klesges RC, Lando H. An examination of cigarette brand switching to reduce health risks. Ann Behav Med. 1999;21:128–134. doi: 10.1007/BF02908293. [DOI] [PubMed] [Google Scholar]
- Hammond D, Fong GT, Cummings KM, Hyland A. Smoking topography, brand switching, and nicotine delivery: results from an in vivo study. Cancer Epidemiol Biomarkers Prev. 2005;14:1370–1375. doi: 10.1158/1055-9965.EPI-04-0498. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Lemmonds C, Tomar S. Smokeless tobacco use: Harm reduction or induction approach? Prev Med. 2004;38:309–317. doi: 10.1016/j.ypmed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson HH. Oral spit tobacco: addiction, prevention and treatment. Nicotine Tob Res. 1999;1:21–44. doi: 10.1080/14622299050011131. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Carmella SG, Chen M, Koch JD, Miller A, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- Hecht SS, Murphy SE, Carmella SG, Li S, Jensen J, Le C, Joseph AM, Hatsukami D. Similar uptake of lung carcinogens by smokers of regular, light, and ultralight cigarettes. Cancer Epidemiol Biomarkers Prev. 2005;14:693–698. doi: 10.1158/1055-9965.EPI-04-0542. [DOI] [PubMed] [Google Scholar]
- Henningfield J, Radzius A, Cone E. Estimation of available nicotine content of six smokeless tobacco products. Tob Control. 1995;4:57–61. [Google Scholar]
- Hughes J, Hatsukami D. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kozlowski L, Goldberg M, Yost B, White E, Sweeney C, Pillitteri J. Smokers' misperceptions of light and ultra-light cigarettes may keep them smoking. Am J Prev Med. 1998;15:78–79. doi: 10.1016/s0749-3797(98)00004-x. [DOI] [PubMed] [Google Scholar]
- Lewin F, Norell S, Johansson H, Gustavsson P, Wennerberg J. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck. Cancer. 1998;82:1367–1374. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Littell R, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Stat Med. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Moyer TP, Charlson JR, Enger RJ, Dale LC, Ebbert JO, Schroeder DR, Hurt RD. Simultaneous analysis of nicotine, nicotine metabolites, and tobacco alkaloids in serum or urine by tandem mass spectrometry, with clinically relevant metabolic profiles. Clin Chem. 2002;48:1460–1471. [PubMed] [Google Scholar]
- National Cancer Institute. Smoking and Tobacco Control Monograph No. 13. 2001. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. [Google Scholar]
- U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, Bethesda, MD. Prue D, Krapfl J, Martin J. Brand fading: the effects of gradual changes to low tar and nicotine cigarettes on smoking rate, carbon monoxide and thiocyanate levels. Behav Ther. 1981;12:400–416. [Google Scholar]
- Richter P, Spierto FW. Surveillance of smokeless tobacco nicotine, pH, moisture, and unprotonated nicotine content. Nicotine Tob Res. 2003;5:885–889. doi: 10.1080/14622200310001614647. [DOI] [PubMed] [Google Scholar]
- Scherer G. Smoking behaviour and compensation: a review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- Schildt E, Eriksson M, Hardell L, Magnusson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer ina Swedish case-control study. Int J Cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Schluchter M. Analysis of incomplete multivariate data using linear models with structured covariance matrices. Stat Med. 1988;7:317–324. doi: 10.1002/sim.4780070132. [DOI] [PubMed] [Google Scholar]
- Severson HH, Hatsukami D. Smokeless tobacco cessation. Prim Care. 1999;26:529–551. doi: 10.1016/s0095-4543(05)70116-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Pillitteri JL, Burton SL, Rohay JM, Gitchell JG. Smokers' beliefs about "Light" and Ultra Light" cigarettes. Tob Control. 2001;10:i17–i23. doi: 10.1136/tc.10.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine Tob Res. 2006;8:309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the Smoke: Assessing the Science Base for Tobacco Harm Reduction. National Academy Press, Institute of Medicine; Washington, DC: 2001. [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Smoking cessation. Agency for Health Care Policy and Research; Rockville, Maryland: 1996. [Google Scholar]


