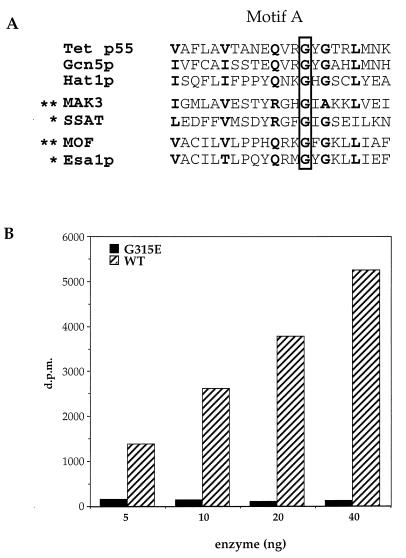
Figure 3.
Point mutant abolishes HAT activity of Esa1p. (A) Alignment of a motif found in a wide variety of acetyltransferases (referred to as domain A in the GNAT superfamily; ref. 7). Note that a specific conserved Gly residue (G315 in Esa1p) has been shown to be necessary for enzymatic function in vitro (marked by ∗) or necessary for gene function in vivo (marked by ∗∗; for a more extensive review, see ref. 7). (B) Various levels of recombinant wild type (WT) and mutant (G315E) Esa1p were assayed for HAT activity with chicken histones. [3H]Acetate incorporation is plotted versus the concentration of enzyme.