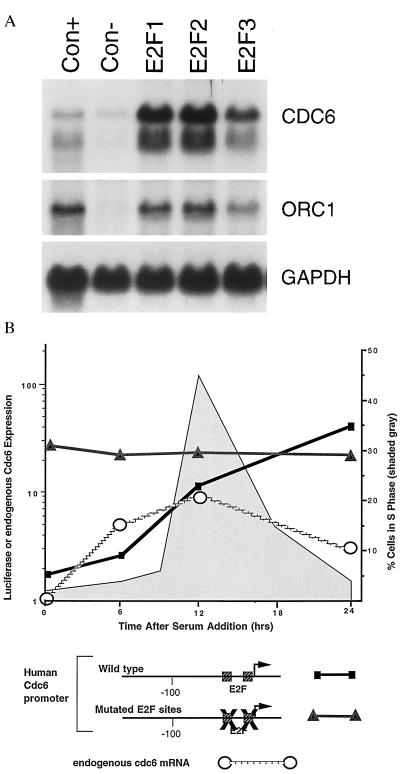
Figure 4.
(A) Quiescent, serum-deprived human foreskin fibroblasts (passage 7) were infected with the indicated recombinant adenoviruses, each at an multiplicity of infection of 300, and then harvested for Northern blot analysis at 15 hr postinfection. Blots were hybridized with a human Cdc6 probe, or with a glyceraldehyde-3-phosphate dehydrogenase probe as a control for RNA loading. The samples labeled as E2F1, E2F2, or E2F3 represent infections with the indicated E2F viruses together with an adenovirus expressing DP1 to provide the dimeric partner for E2F. (B) E2F binding sites are required for correct regulation of the human Cdc6 promoter. Transient transfection assays were performed in subconfluent 3T3 cells under conditions of growth arrest induced by serum deprivation (t = 0 hr), and at subsequent time points after readdition of serum. Reporter gene activity (mean of triplicate determinations) was calculated relative to expression of a cytomegalovirus-luciferase gene in sister cultures assayed at each time interval, and displayed in relation to endogenous Cdc6 mRNA (Northern blot analysis; calculated as multiples of the hybridization signal at t = 0 hr) and to the fraction of cells in S phase (flow cytometry). Similar results were obtained in each of four separate transfection assays. A schematic representation of the human Cdc6 promoter illustrates E2F binding sites in relation to the transcriptional start site (arrow) of the human Cdc6 gene (14), and disruption of these sites (X) in the mutated promoter.