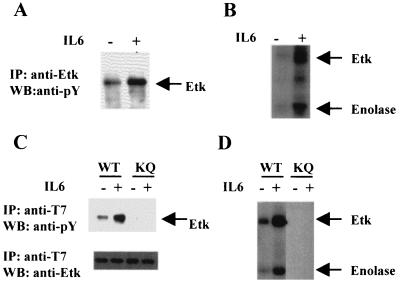
Figure 2.
Activation of Etk by IL-6 in LNCaP and CV-1 cells. (A) IL-6 induces the tyrosine phosphorylation of Etk in LNCaP. The cell extracts from the IL-6-treated(+) or untreated (−) cells were subjected to immunoprecipitation by the antiserum against Etk respectively, followed by Western blot analysis with anti-phosphotyrosine antibody. (B) IL-6 stimulates the kinase activity of Etk in LNCaP. The Etk immunoprecipitates from A were subjected to in vitro kinase assay with enolase as a substrate. The phosphorylated Etk and exogenous substrate enolase are indicated. (C) IL-6 induces tyrosine phosphorylation of exogenous T7-tagged wild-type Etk (WT), but not EtkK444Q mutant (KQ) in CV-1 cells. The CV-1 cells were transfected by pcDNA3-T7-Etk (wild type) or pcDNA3-T7-EtkKQ, respectively. The cell extracts from the IL-6-treated (+) or untreated (−) cells were subjected to immunoprecipitation by anti-T7 antibody respectively, followed by Western blot analysis with antiphosphotyrosine antibody. (D) IL-6 stimulates the in vitro kinase activity of Etk in CV-1 cells. The T7 immunoprecipitates from C were subjected to in vitro kinase assay. The phosphorylated Etk and exogenous substrate Enolase are indicated.