Figure 2. Overall structure of E. coli AnsA.
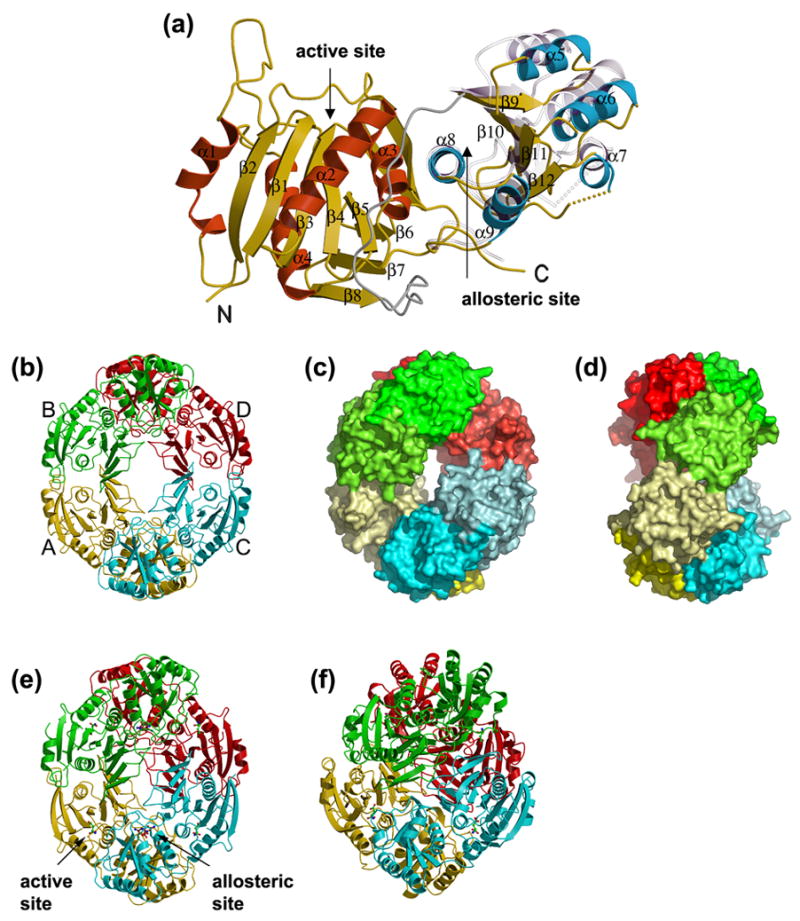
(a) A ribbon representation of the apo AnsA monomer showing the secondary structure elements. The N-terminal domain α-helices are shown in red, the C-terminal domain α-helices are blue and the β-strands are in yellow in both domains of the molecule. The linker region is shown in grey. Shown semi-transparently is the position of the C-terminal domain relative to the fixed N-terminal domain in the structure of AnsA complexed with L-asparagine. Note that the movement is a rotation around helix α8 at the domain interface. (b–d) The tetrameric structure of apo AnsA with monomers A, B, C and D (labeled in b) in yellow, green, cyan and red, respectively. (b) A ribbon representation; (c) a surface representation showing the donut shape with the hole in the center; (d) a 90° rotation of (c) showing the tight (top) and loose (front, middle) interfaces. In (b) and (c), the N- and C-terminal domains are shaded differently for clarity. (e) The tetrameric structure of AnsA complexed with L-asparagine in a ribbon representation showing aspartate (green) at the four active sites and asparagine (brown) at the four allosteric sites (one of each site is indicated). Compared to the apo structure (b), the tetramer is more compact and the central hole is smaller. (f) The tetrameric structure of E. coli AnsB in ribbon representation in which the central hole is absent due to a more orthogonal packing of tight dimers.
